Higher activity of the protein PGC-1α enables brown fat cells in females to engage in thermogenic activity and energy expenditure more effectively than in males, according to a groundbreaking study conducted in Japan. The research, led by a team from the Institute of Science Tokyo, reveals that PGC-1α promotes phospholipid synthesis, strengthening the mitochondria of brown fat cells and enhancing their heat-generating capacity in female mice. These findings could inspire new therapies for the prevention of obesity and diabetes.
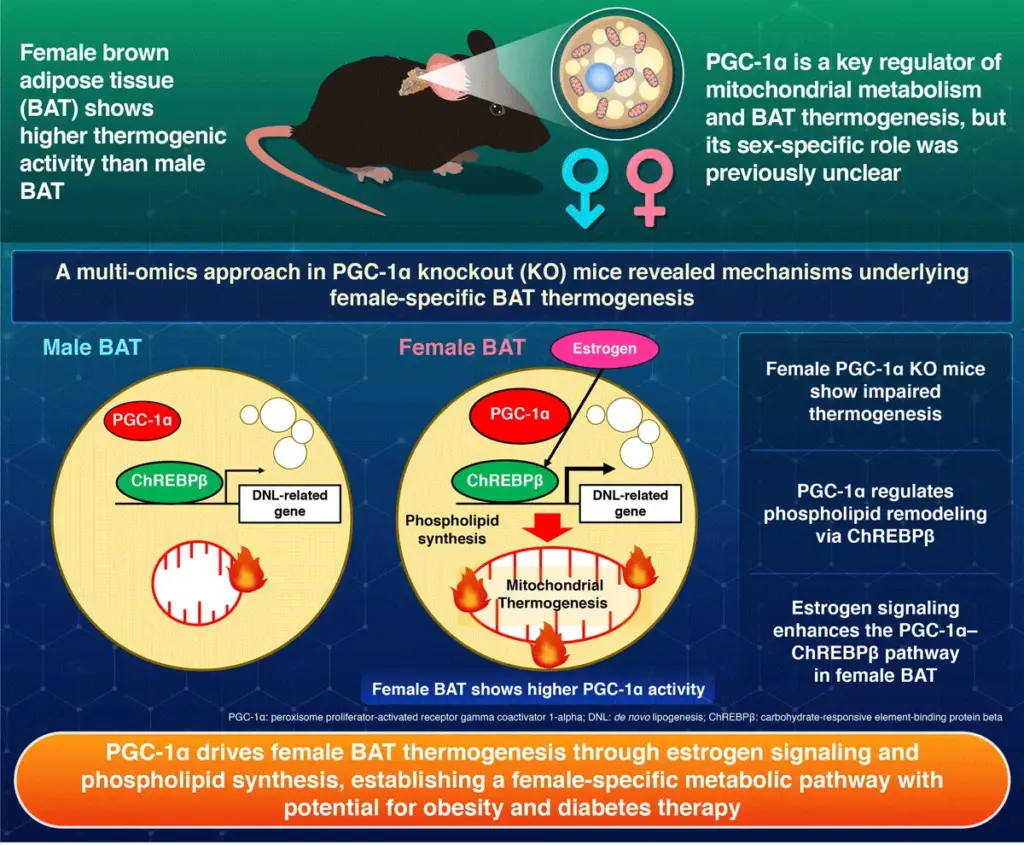
Obesity remains a significant global health concern, contributing to diabetes and a range of metabolic disorders. Interestingly, while obesity affects both sexes, women are generally less prone to obesity-related diabetes and cardiovascular diseases compared to men. The biological reasons for this difference have been unclear, but brown adipose tissue (BAT) has emerged as a potential factor. BAT is a specialized fat tissue that dissipates energy as heat to maintain body temperature. Previous studies have shown that BAT is more metabolically active in females than in males, but the exact molecular mechanism has remained elusive.
Unraveling the Mechanism
To address this question, a research team led by Assistant Professor Kazutaka Tsujimoto, graduate students Akira Takeuchi and Jun Aoki, and Professor Tetsuya Yamada from the Department of Molecular Endocrinology and Metabolism, Graduate School of Medical and Dental Sciences, Science Tokyo, in collaboration with colleagues from the University of Tokyo, set out to investigate the mechanism underlying the sex-specific activity of BAT. Their findings were published in the journal Nature Communications on July 14, 2025.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a key regulator of energy metabolism and mitochondrial activity, found in tissues including brown fat, heart, skeletal muscle, and brain. “PGC-1α is a master regulator of mitochondrial function in BAT,” explains Yamada and Tsujimoto. “So, to uncover the sex-specific mechanism of BAT, we focused on the activity of PGC-1α.”
Methodology and Findings
Using genetically modified mice that lacked PGC-1α protein only in BAT cells, the team compared male and female mice using multi-omics approaches, including transcriptomics, metabolomics, and lipidomics, to elucidate the protein’s role in detail. According to the results, removing PGC-1α impaired BAT thermogenesis only in female mice, as evidenced by their lower body temperature during cold exposure. Additionally, they showed reduced oxygen consumption, and their mitochondria had fewer and less organized cristae—the internal folds where energy production occurs.
“This coordination between PGC-1α and estrogen explains why female BAT outperforms male BAT in energy expenditure,” says Yamada and Tsujimoto.
Molecular profiling revealed key insights into this mechanism: PGC-1α activates genes involved in de novo lipogenesis (DNL), in part through carbohydrate-response element-binding protein beta (ChREBPβ)—a transcription factor that regulates expression of DNL-related genes. This pathway boosts the production of certain phospholipids, including ether-linked phosphatidylethanolamine and cardiolipin, which are essential for maintaining mitochondrial structure and function. Without these lipids, mitochondria become less efficient, reducing the tissue’s ability to generate heat.
Implications for Future Therapies
The study’s findings hold significant implications for the development of new therapies targeting obesity and metabolic disorders. The PGC-1α-ChREBPβ lipid synthesis pathway, further enhanced by estrogen signaling, could be an entirely new target for therapies to enhance lipid metabolism. To support this, the researchers conducted additional experiments showing that suppressing ChREBPβ in female BAT reproduced the same mitochondrial defects and reduced thermogenesis observed with PGC-1α deletion. This effect was not observed in males, highlighting the sex-specific characteristic of the mechanism.
“Stimulating this pathway could promote energy expenditure, improve metabolic health, and prevent obesity and type 2 diabetes,” the researchers suggest.
Overall, the study provides new insight into how biological sex shapes energy metabolism, identifying PGC-1α-mediated phospholipid synthesis as a key regulator of BAT thermogenesis. The findings set the stage for new interventions based on metabolic mechanisms, paving the way toward a healthier future.
Looking Ahead
As the world grapples with rising obesity rates, these insights into the sex-specific mechanisms of fat metabolism could lead to more personalized and effective treatments. Future research may explore how these findings can be translated into human therapies, potentially offering new hope in the fight against obesity and its associated diseases. The discovery of the PGC-1α and estrogen interplay in energy metabolism underscores the importance of considering biological sex in medical research and treatment development.