Tiny structures inside human cells, known as mitochondria, are crucial for converting food into energy, powering every heartbeat, thought, and movement. When these structures fail, organs that require the most energy, such as the heart, brain, and muscles, are the first to suffer. Many serious diseases trace back to mitochondrial breakdown, yet most treatments today only alleviate symptoms without addressing the root energy deficit.
Now, a groundbreaking method developed by scientists at Texas A&M University could change this paradigm. The team has discovered a way for healthy cells to donate surplus mitochondria to their damaged neighbors, potentially restoring cellular power where it has diminished. This research, published in the Proceedings of the National Academy of Sciences (PNAS), marks a significant step forward in cellular repair.
A Natural Repair System, Supercharged
Cells naturally share mitochondria in small amounts, with stem cells particularly adept at this rescue work. However, the process occurs too infrequently to serve as an effective treatment. The researchers at Texas A&M posed a pivotal question: What if donor cells could be enhanced to provide more mitochondria?
To explore this, they utilized flower-shaped nanoparticles made from molybdenum disulfide. These “nanoflowers” act like sponges, absorbing harmful oxygen molecules that accumulate under stress. This reduction in oxidative stress triggers a cascade of cellular reactions, leading to increased mitochondrial growth.
In laboratory tests, stem cells treated with these nanoflowers doubled their mitochondrial DNA within seven days, indicating a significant increase in functional mitochondria. Importantly, this process did not involve drugs or genetic modifications.
“We have trained healthy cells to share their spare batteries with weaker ones,” said biomedical engineer Akhilesh Gaharwar. “By increasing the number of mitochondria inside donor cells, we can help aging or damaged cells regain their vitality, without any genetic modification or drugs.”
How the Boost Works
The nanoflowers reduce reactive oxygen species, harmful molecules that can damage cells. This reduction activates a protective enzyme called SIRT1, which in turn triggers PGC-1α, a master switch for energy growth. Once activated, the cell rapidly begins producing new mitochondria.
The researchers tested particles ranging from 100 to 250 nanometers in size, finding that the smallest particles penetrated cells more easily and were effective at lower doses. Importantly, these particles did not harm cell growth or health at safe levels.
Passing Power Through Cell Tunnels
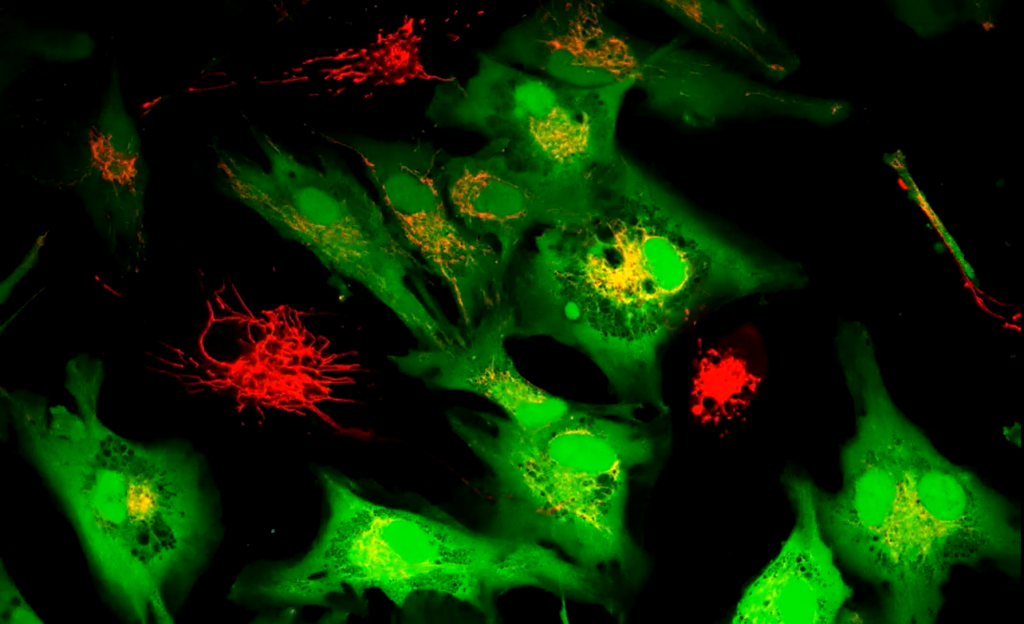
Next, the scientists observed interactions between these supercharged stem cells and injured cells. When placed side by side, tiny bridges known as tunneling nanotubes formed between them, allowing mitochondria to transfer from stem cells to heart, muscle, and smooth muscle cells.
The results were remarkable. Treated stem cells delivered about twice as many mitochondria to muscle cells, with transfers to heart and smooth muscle cells increasing three to four times. Blocking the growth of these tunnels halted the transfer, confirming that mitochondria moved through direct contact rather than the surrounding fluid.
Energy Returns
The effectiveness of the transferred mitochondria was evident in the recipient cells’ energy levels. ATP, the primary cellular fuel, increased, and oxygen consumption rose, indicating active mitochondria. The energy boost did not result from changes in sugar metabolism, but from the new mitochondria.
Genetic analysis revealed that while mitochondrial genes remained unchanged, nuclear genes involved in energy production and mitochondrial maintenance became more active, strengthening key energy systems.
Healing Damaged Cells
The team tested the method on cells exposed to toxins that damage mitochondria, including doxorubicin, a chemotherapy drug known to harm the heart. In each case, donated mitochondria helped reverse damage, restoring ATP levels, reducing harmful molecules, and improving electrical balance.
Heart cells damaged by chemotherapy showed significant recovery, with decreased signals of programmed cell death and restored healthy energy use, demonstrating the potential of this technique.
From Lab Dish to Living Patients
The concept of delivering energy rather than just medicine could revolutionize treatment for conditions like heart disease and muscle loss. Future therapies might involve preparing stem cells with nanoflowers for transplantation or targeting therapy to weakened tissues. The particles can be modified to direct treatment to specific organs, such as the heart or brain.
However, the researchers emphasize caution. Animal testing is ongoing, and human studies will take time. Researchers need to determine the best delivery methods, safe dosages, and the duration of benefits.
“This is an early but exciting step toward recharging aging tissues using their own biological machinery,” Gaharwar stated. “If we can safely boost this natural power-sharing system, it could one day help slow or even reverse some effects of cellular aging.”
Currently, the research remains in the laboratory, but for diseases linked to energy failure, it offers a rare opportunity to address the underlying issue rather than just the symptoms.
Practical Implications of the Research
This research could pave the way for new treatments for heart disease, muscle disorders, and conditions associated with aging and low cellular energy. By restoring power within diseased cells, future therapies may slow damage instead of merely alleviating pain or loss of function.
The approach could also mitigate side effects from heart-damaging cancer drugs and lead to therapies that enable tissues to heal themselves by sharing healthy mitochondria.
These findings are available in the journal PNAS.
For further reading, consider related stories such as “Space travel accelerates stem cell aging – threatening astronaut health,” “New drug Rapalink-1 slows cellular aging, study finds,” and “New protein discovery opens the door to slowing or even reversing cellular aging.”