In a groundbreaking study published on October 9 in Nature Chemical Biology, researchers from the University of Washington have unveiled a novel approach to targeted drug delivery using programmable proteins. These proteins, equipped with autonomous decision-making capabilities, promise to revolutionize how therapies are delivered, ensuring that medicines reach only the specific areas of the body where they are needed.
Targeted drug delivery is a burgeoning field in medicine, offering the potential to reduce dosages and minimize harmful side effects by directing therapies precisely where they are needed. For instance, targeted immunotherapies can hone in on cancerous tissues, activating immune cells to combat the disease exclusively in those areas.
Breakthrough in Protein Design
The challenge has always been to create “smart” therapies capable of navigating the body and determining their targets autonomously. The University of Washington team, led by Professor Cole DeForest, has made significant strides in this direction. By integrating smart tail structures into therapeutic proteins, they have enabled these proteins to respond to specific environmental cues, thereby controlling their localization within the body.
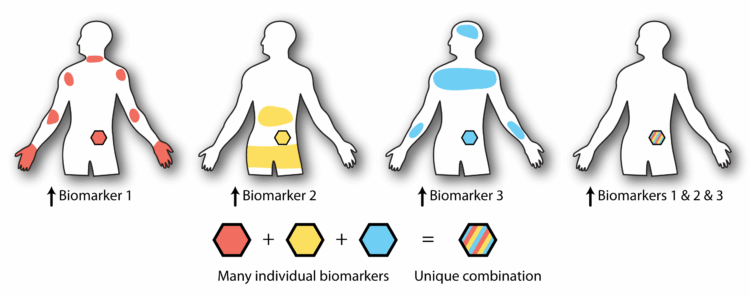
The innovative protein tails are designed to fold into predetermined shapes, dictating their reactions to various combinations of biomarkers. This allows for the precise targeting of therapies, reducing the risk of off-target effects.
“We’ve now finally figured out how to produce these systems faster, at scale and with dramatically enhanced logical complexity,” said Cole DeForest, senior author of the study.
Advancements in Synthetic Biology
The development of these programmable proteins has been facilitated by advances in synthetic biology, which have enabled the rapid and cost-effective production of complex proteins. This represents a departure from traditional methods, which were time-consuming and labor-intensive.
According to Murial Ross, a doctoral student of bioengineering and co-first author of the study, “The field has developed exciting new protein-based tools that can allow researchers to form permanent bonds between proteins, opening doors for new protein structures that were previously unachievable.”
These advancements have allowed DeForest’s team to create proteins with sophisticated logical circuits capable of responding to multiple biomarkers. This innovation is a significant leap forward from their 2018 work, which introduced materials responsive to multiple biomarkers using Boolean logic.
Implications for Future Therapies
The implications of this research are profound. By designing proteins that can respond to up to five different biomarkers, the team has opened the door to more precise and effective therapies. These proteins can be attached to various carriers, such as hydrogels or living cells, for targeted delivery to specific sites within the body.
DeForest envisions a future where these technologies could be applied to a wide range of medical applications, from cancer treatments to diagnostic tools. “The dream is to be able to pick any arbitrary location inside of the body – down to individual cells – and program a material to go and act there,” he explained.
“The sky’s the limit. You can create delayed and independent delivery of many different components in one treatment,” added Murial Ross.
Next Steps and Future Research
Looking ahead, the research team plans to explore additional biomarkers that could be targeted by these programmable proteins. They also aim to collaborate with other laboratories to develop real-world therapies based on their findings.
Co-authors of the study include Ryan Gharios, Annabella Li, Shivani Kottantharayil, and Jack Hoye, all affiliated with the University of Washington. The research was funded by the National Science Foundation and the National Institutes of Health.
This breakthrough in programmable proteins marks a significant step towards more personalized and precise medical treatments, with the potential to transform the landscape of targeted drug delivery.