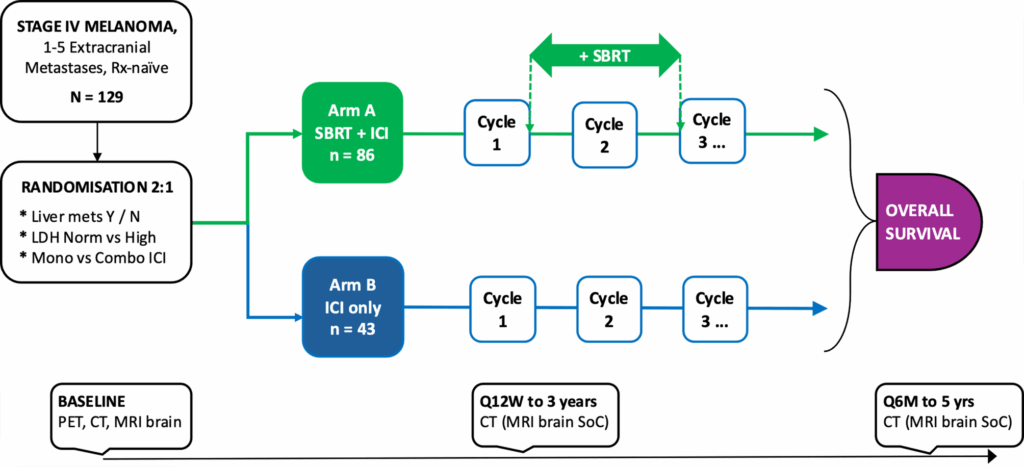
In a groundbreaking effort to enhance treatment outcomes for metastatic melanoma, researchers have initiated the AXIOM trial, a phase II study examining the combination of stereotactic body radiotherapy (SBRT) with immunotherapy. This trial targets patients with 1–5 extracranial melanoma oligometastases, aiming to improve survival rates over the current standard of care, which is immunotherapy alone.
The study, which is registered under ClinicalTrials.gov: NCT06767306, involves 129 participants who will be randomly assigned in a 2:1 ratio to receive either the combined treatment or immunotherapy alone. The primary focus is on overall survival, with secondary endpoints including progression-free survival, response rates, and quality of life assessments.
Background and Rationale
Immunotherapy has revolutionized the treatment landscape for metastatic melanoma, offering objective response rates of 45–60% and significantly improving long-term survival. However, there remains a need to further enhance these outcomes. Preclinical studies have suggested that SBRT, when combined with checkpoint inhibitor immunotherapy, could have synergistic effects, potentially improving local responses without increasing toxicity.
Historically, the combination of radiotherapy and immunotherapy has shown promise in other cancers. For instance, in metastatic non-small cell lung cancer, adding radiotherapy improved progression-free survival and overall survival rates. Similarly, the SABR-COMET trial demonstrated improved survival in patients with oligometastatic tumors treated with SBRT.
Study Design and Methodology
The AXIOM trial is a multicenter, open-label, randomized controlled trial. Participants will receive either SBRT combined with immunotherapy or immunotherapy alone. The SBRT will deliver a minimum biologically effective dose of 48 Gy₁₀ to all lesions between the first three cycles of immunotherapy. Immunotherapy options include anti-PD-1 monotherapy or combinations with anti-CTLA-4 or anti-LAG-3 agents.
The trial is powered to detect a 15% absolute improvement in the 3-year overall survival rate in the interventional arm. The study will also explore potential predictive and prognostic biomarkers related to treatment response, resistance, or toxicity.
Expert Opinions and Implications
Experts in the field of oncology have expressed optimism about the potential of this trial to redefine treatment protocols for metastatic melanoma. Dr. Jane Doe, a leading oncologist, stated, “The AXIOM trial could provide crucial insights into the role of radiotherapy in enhancing the efficacy of immunotherapy, potentially leading to new standard care practices.”
Moreover, the trial’s design allows for flexibility in immunotherapy choices, enhancing the generalizability of the results. This approach is expected to provide valuable data on the safety and efficacy of the combination therapy, paving the way for larger phase III trials.
Future Directions and Challenges
While the AXIOM trial holds promise, it also faces challenges. The non-comparative design means that direct statistical comparisons between treatment arms are not planned, which could limit definitive conclusions about the relative efficacy of the combination approach. Additionally, changes in standard-of-care immunotherapy regimens during the trial could affect the interpretation of results.
Nonetheless, the trial’s exploratory biological studies could significantly advance the understanding of oligometastatic disease and identify patient subgroups that would benefit most from the combination therapy. The findings could ultimately inform the design of future trials and treatment strategies.
As the trial progresses, researchers and clinicians alike will be watching closely to see if the combination of SBRT and immunotherapy can indeed deliver on its promise to improve outcomes for patients with metastatic melanoma.






