(MEMPHIS, Tenn. – August 27, 2025) A groundbreaking study from St. Jude Children’s Research Hospital has unveiled new insights into how biomolecular condensates, formed by an abnormal fusion protein, could be pivotal in developing new treatments for ependymoma, the third most common childhood brain tumor. The research, published today in Nature Cell Biology, focuses on the fusion protein ZFTA–RELA, which is implicated in 95% of ependymomas in the brain cortex.
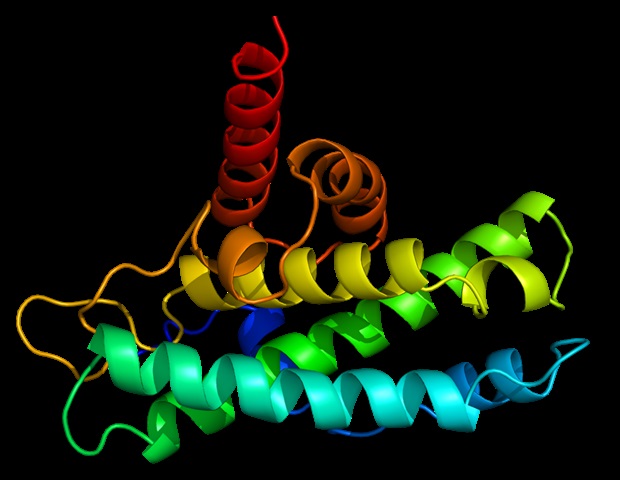
The study reveals that disordered regions of the ZFTA–RELA fusion protein lead to the formation of cellular droplets known as condensates. These “membraneless organelles” are essential for the development of ependymoma, providing a potential target for therapeutic intervention. “Ependymomas have had essentially the same treatments for 30 years,” said co-corresponding author Dr. Stephen Mack of the St. Jude Department of Developmental Neurobiology. “By describing how this abnormal fusion protein drives this deadly brain tumor, we are paving the road to look for new targeted therapies.”
Exploring the Role of Fusion Proteins
The researchers began their investigation by dissecting the different regions of the ZFTA–RELA fusion protein. They discovered that the ZFTA portion, which directly binds to DNA, is crucial for cancer development. Meanwhile, the RELA portion, characterized by a highly disordered region, is necessary for condensate formation. These condensates organize molecules within cells, facilitating various functions.
In experiments, when the disordered RELA region was removed, condensates did not form, and ependymoma did not develop in mice. However, when the RELA portion was replaced with other disordered protein regions, the new fusions still formed condensates, activating oncogene expression and leading to tumor development.
“Our findings strengthen the view that condensate formation should be considered as a driving mechanism for oncogenic fusion proteins in general,” said co-corresponding author Dr. Richard Kriwacki of the St. Jude Department of Structural Biology. “Especially for those that alter chromatin biology.”
Condensates: A New Therapeutic Frontier
In ependymoma, condensates gather molecules responsible for gene expression. Within these structures, the ZFTA portion of the fusion protein directs the complex to DNA sequences encoding oncogenes. This abnormal process is essential for ependymoma formation, suggesting a new therapeutic target.
“Fusion proteins such as ZFTA–RELA are challenging drug targets, but our findings provide an indirect approach,” Kriwacki added. “Instead of focusing on this fusion protein, we can now start identifying its interacting partners within condensates, examining which are essential for tumor formation and targeting those.”
While the research focused on ependymoma, it opens doors to exploring similar vulnerabilities in other cancers driven by fusion proteins. “We discovered a novel mechanism for assembling molecules that underlies the formation of a deadly brain tumor,” Mack said. “By understanding these aberrant condensates, we may have found a new place to look for therapeutic interventions for cancers driven by fusion oncoproteins.”
Implications and Future Directions
The implications of this study are profound, suggesting that targeting the components of condensates could revolutionize treatment strategies for ependymoma and potentially other cancers. The ability to disrupt the formation of these condensates could lead to innovative therapies that are more effective and less toxic than current options.
As researchers continue to unravel the complexities of fusion proteins and their role in cancer, the focus will likely shift towards identifying and targeting the molecular interactions within condensates. This could pave the way for precision medicine approaches that tailor treatments to the specific molecular characteristics of a patient’s tumor.
Authors and Funding
The study’s co-first authors include Amir Arabzade, Hazheen K. Shirnekhi, and Srinidhi Varadharajan from St. Jude, among others. The research was supported by grants from the National Cancer Institute, the Department of Defense, the National Institutes of Health, and several foundations dedicated to pediatric cancer research.
This pioneering work underscores the importance of continued investment in cancer research and the potential for scientific breakthroughs to transform patient care. As the scientific community builds on these findings, the hope is to develop more effective treatments that offer new hope to children and families affected by ependymoma and similar cancers.