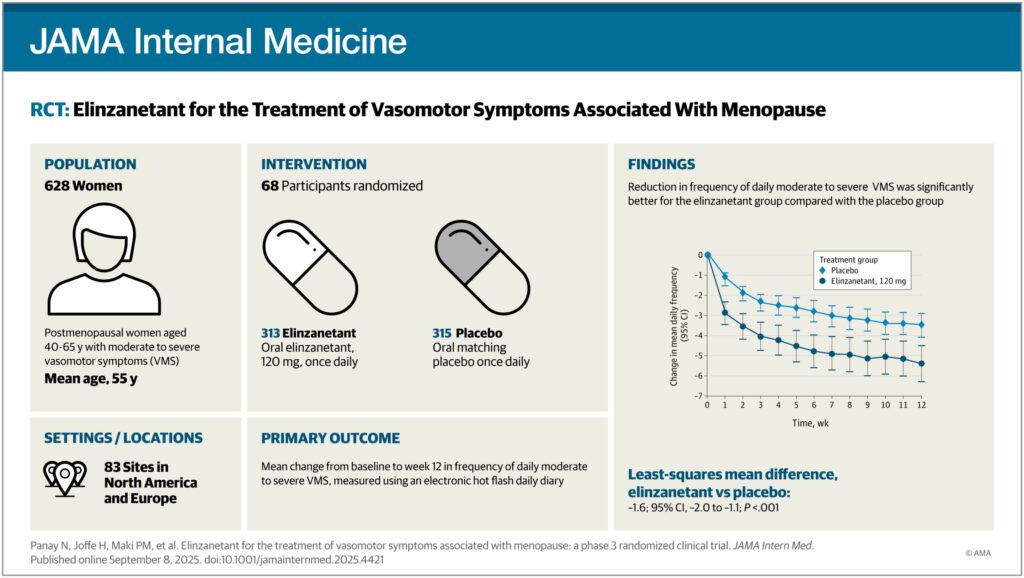
The investigational drug elinzanetant has demonstrated a significant reduction in hot flashes and night sweats among postmenopausal women, according to a comprehensive international clinical trial. The OASIS-3 trial, which enrolled over 600 women aged 40 to 65 across 83 sites in North America and Europe, revealed that participants taking 120 mg of elinzanetant daily experienced a more than 73% reduction in the frequency and severity of vasomotor symptoms by week 12.
This promising development comes as many women seek alternatives to hormone therapy, which can carry risks such as breast tenderness, headaches, and an increased risk of certain cancers. Elinzanetant, a nonhormonal drug, offers a potential new option for those unable or unwilling to undergo traditional hormone treatments.
Clinical Trial Insights and Findings
The OASIS-3 trial’s findings are particularly significant given the scale and duration of the study. Participants were monitored over a 52-week period, with results indicating not only a rapid reduction in symptoms but also sustained relief over the course of a year. The trial also noted secondary benefits, including reduced sleep disturbances and improved quality of life, although these aspects require further study to be fully understood.
Importantly, the trial found no harmful effects on liver function or bone density, a common concern with many medications. “Elinzanetant, the first dual neurokinin-1 and 3 receptor antagonist to complete Phase 3 testing, has shown promising results,” said Dr. JoAnn V. Pinkerton, a leading researcher involved in the study and director of midlife health at UVA Health.
For those dealing with moderate to severe VMS due to menopause, the treatment options have been limited, especially for those who cannot or choose not to undergo hormone therapy.
Understanding Elinzanetant’s Impact
Elinzanetant’s development is a response to the need for effective non-hormonal treatments for menopause symptoms. Traditional hormone therapy, while effective for some, is not suitable for all women, particularly those with a history of blood clots or cancer. Elinzanetant, devoid of estrogen, could fill this gap by providing relief without the associated risks of hormone replacement therapies.
Previous trials, OASIS-1 and OASIS-2, had already indicated the drug’s potential, but OASIS-3’s larger scale and extended duration have provided more robust evidence of its efficacy and safety. “Outcomes from this 52-week study were consistent with those observed in the 26-week OASIS-1 and OASIS-2 trials,” researchers noted in their published findings.
Common side effects reported included sleepiness, fatigue, and headaches, yet no significant safety concerns were identified through mammograms or measures of endometrial thickness.
Future Implications and Regulatory Path
The potential approval of elinzanetant by the FDA could mark a significant advancement in menopause treatment. However, the FDA has delayed its decision, requesting additional time to review Bayer’s application, which includes data from the OASIS-3 trial. This delay underscores the rigorous process involved in bringing new pharmaceuticals to market.
Meanwhile, the OASIS-4 trial is exploring elinzanetant’s efficacy in women experiencing hot flashes due to breast cancer endocrine therapy, with initial results mirroring those of the OASIS-3 trial. “What is so exciting is that with elinzanetant, we potentially have a new treatment option that can be used first-line for moderate to severe hot flashes,” Dr. Pinkerton remarked.
The continued research and eventual approval of elinzanetant could provide much-needed relief for millions of women worldwide, offering a new avenue for managing the often debilitating symptoms of menopause.