The mitochondria, often dubbed the powerhouse of the cell, may have a more sinister role in cancer progression. New research highlights the mitochondrial metabolite glutathione as a key player in aiding breast cancer cells to break away from primary tumors, traverse the body, and establish themselves in new tissues. This discovery sheds light on the intricate processes of metastasis, offering potential new avenues for cancer treatment and research.
The study, led by Kivanç Birsoy at Rockefeller University’s Laboratory of Metabolic Regulation and Genetics, is among the first to connect a specific mitochondrial metabolite to cancer metastasis. “We hope that our work will bring more attention to how organelles and their metabolites are relevant to cancer biology,” Birsoy stated.
A Mysterious Connection with Metastasis
Metastasis is responsible for the majority of cancer-related deaths, rather than the primary tumor itself. Researchers have long sought to understand the factors that enable cancer cells to spread throughout the body. Metabolites, the small molecules involved in metabolism, have been shown to play crucial roles in various stages of metastasis.
Previous studies have identified metabolites such as lactate, pyruvate, glutamine, and serine as important in cancer spread. Given that mitochondria are responsible for energy generation and metabolite production, their connection to metastasis is not surprising. However, pinpointing the exact mechanisms has proven challenging. “Mitochondria have thousands of metabolites, and it’s been difficult to determine which are important to tumor formation and growth, and which initiate metastasis,” Birsoy explained.
Cells Under Stress
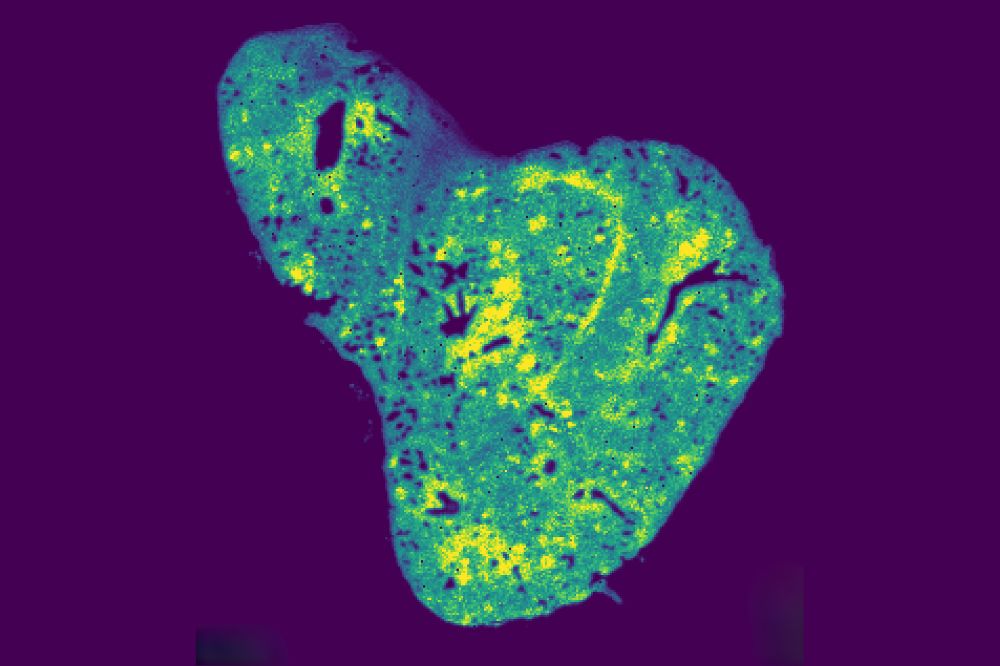
In their innovative study, Birsoy and his team used protein tagging to differentiate between primary tumor cells and those that had migrated from the breast to the lung. This allowed them to analyze how mitochondrial metabolites change as cancer cells colonize new sites. “These techniques allowed us to, in an unbiased manner, see the difference between what’s essential in metastasis and what’s essential in the primary tumor,” noted Nicole DelGaudio, a graduate fellow involved in the study.
Among the thousands of mitochondrial compounds, glutathione stood out. Known for its role as a major antioxidant, glutathione levels were found to be significantly elevated in metastatic cancer cells in the lung. Further analysis using spatial metabolomics confirmed the distribution of glutathione within lung tissues.
The researchers then focused on mitochondrial membrane proteins, identifying SLC25A39 as a crucial transporter for metastatic cells in the lung. This discovery linked glutathione and its transporter to metastasis, demonstrating that mitochondrial glutathione import via SLC25A39 is essential for cancer spread.
“Because we found this transporter earlier and knew how to block the entry of glutathione, we already had the tools necessary to investigate its role in cancer metastasis,” Birsoy said.
A Familiar Culprit
This research builds on previous work by Birsoy’s lab. In 2021, they identified SLC25A39 as the transporter for glutathione into mitochondria. By 2023, they showed that SLC25A39 not only transports but also regulates glutathione levels within mitochondria. When this metabolite and its transporter appeared in cancer screenings, Birsoy knew the direction for further experiments.
The implications of these findings are significant. Breast cancer samples from patients with lung metastasis showed elevated SLC25A39 levels, which correlated with poorer overall survival rates. This suggests that targeting this metabolite and its transporter could potentially prevent breast cancer metastasis with fewer side effects than broader therapies.
Clinical Implications and Future Research
While these findings offer promising new targets for cancer treatment, the study emphasizes the need for a deeper understanding of how metabolites function within different cellular compartments. “We’re trying to make our knowledge of metabolism more precise,” Birsoy emphasized. “It’s not just about some metabolite levels going up and others going down. We need to look at the organelles, the precise compartments, to understand how metabolites influence human health.”
The research opens the door for developing small molecules that block the SLC25A39 transporter, potentially halting breast cancer metastasis. However, further studies are necessary to explore the full potential of these findings and their application in clinical settings.
As the scientific community continues to unravel the complexities of cancer metastasis, the role of mitochondria and their metabolites remains a critical area of focus. This study not only advances our understanding of cancer biology but also highlights the intricate balance of cellular processes that can be manipulated for therapeutic benefit.






