For decades, synthetic biologists have been working on gene circuits that can be introduced into cells for various applications, such as reprogramming stem cells into neurons or producing proteins to treat diseases like fragile X syndrome. These circuits are typically delivered via nonpathogenic viruses. However, ensuring that cells produce the correct amount of protein encoded by synthetic genes has been challenging.
To address this issue, MIT engineers have developed a novel control mechanism that allows them to set and adjust the desired protein level for any gene circuit. This innovative approach also enables editing of the set point after the circuit is implemented. “This is a really stable and multifunctional tool. The tool is very modular, so there are a lot of transgenes you could control with this system,” said Katie Galloway, an assistant professor in Chemical Engineering at MIT and the senior author of the study.
Dialing Up Gene Expression
Synthetic gene circuits are designed to include the gene of interest and a promoter region where transcription factors and other regulators can bind, activating the expression of the synthetic gene. However, achieving uniform gene expression across a cell population has proven difficult. Variability arises because some cells may incorporate a single copy of the circuit, while others take up multiple copies, and natural variations in protein production also play a role.
The researchers devised a method to control gene expression levels by altering the distance between the synthetic gene and its promoter. They discovered that a longer DNA “spacer” between the promoter and the gene results in lower gene expression, as it reduces the likelihood of transcription factors effectively activating gene transcription.
To create adjustable set points, the team incorporated sites within the spacer that can be excised by an enzyme called Cre recombinase. Removing parts of the spacer brings transcription factors closer to the gene, increasing gene expression. The researchers developed spacers with multiple excision points, each targeted by different recombinases, creating a system called DIAL. This system allows for “high,” “med,” “low,” and “off” set points for gene expression.
Reprogramming Cells
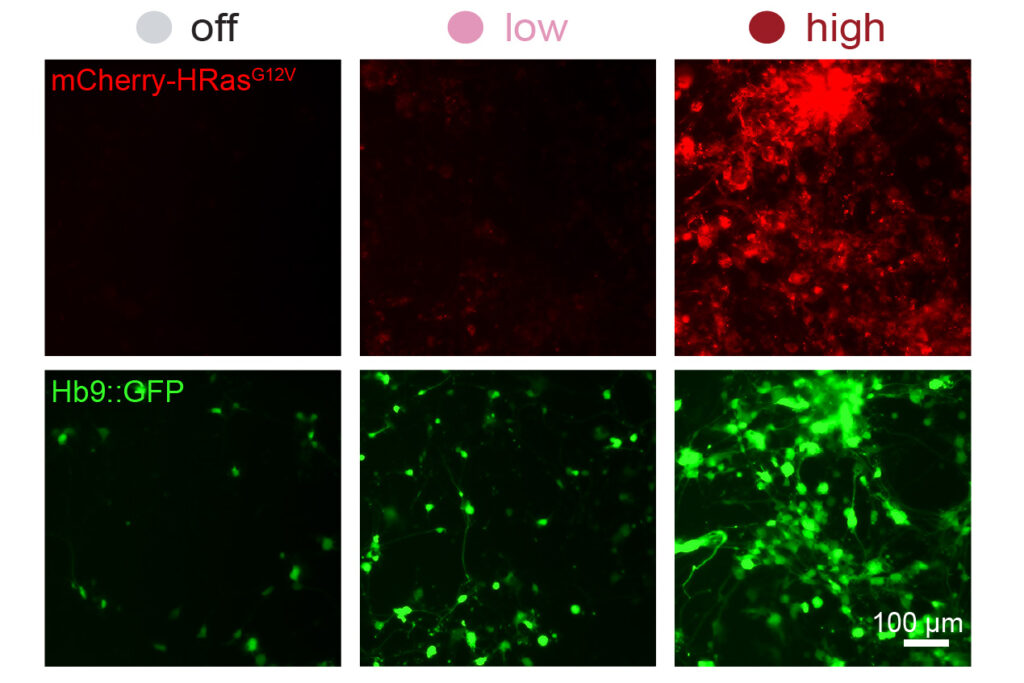
To demonstrate the potential applications of the DIAL system, the researchers used it to deliver varying levels of the gene HRasG12V to mouse embryonic fibroblasts. This HRas variant has been shown to enhance the conversion rate of fibroblasts to neurons. The MIT team found that cells receiving a higher dose of the gene were more likely to successfully transform into neurons.
Researchers now aim to conduct systematic studies of different transcription factors that can induce cellular transitions to various cell types. These studies could reveal how different factor levels affect success rates and whether altering transcription factor levels might change the resulting cell type.
Combining Systems for Tailored Therapies
In ongoing work, the researchers have demonstrated that DIAL can be combined with a previously developed system known as ComMAND, which uses a feedforward loop to prevent cells from overexpressing therapeutic genes. By using these systems together, it may be possible to customize gene therapies to produce specific, consistent protein levels in the target cells of individual patients.
“This is something we’re excited about because both DIAL and ComMAND are highly modular, so you could not only have a well-controlled gene therapy that’s somewhat general for a population, but you could, in theory, tailor it for any given person or any given cell type,” Galloway explained.
Implications and Future Directions
The development of this system represents a significant advancement in synthetic biology, offering a more precise method for controlling gene expression. This could have far-reaching implications for gene therapy, regenerative medicine, and biotechnology. By providing a stable and uniform control mechanism, researchers can build more reliable systems, overcoming one of the major limitations in the field.
The research was funded by the National Institute of General Medical Sciences, the National Science Foundation, and the Institute for Collaborative Biotechnologies. As the technology progresses, it holds the promise of revolutionizing how synthetic genes are used in medical and research applications, potentially leading to more effective treatments for a variety of diseases.