In a groundbreaking study published in Science Advances, researchers from the University of California, Davis have tracked the movement of fluorescent particles within the cells of microscopic worms. This research provides unprecedented insights into the phenomenon of cellular crowding in multicellular organisms, revealing a level of complexity previously unseen in single-celled yeast or mammalian tissue culture cells.
The study found that the cytoplasm inside these worms is significantly more crowded and compartmentalized. This discovery underscores the necessity of studying cellular processes in living organisms rather than relying solely on cell cultures, according to Xiangyi Ding, the study’s first author and a Ph.D. candidate in the Integrative Genetics and Genomics graduate group.
“This changes everything. Crowding in a cell affects any process that depends on molecule movement and interaction, including drug delivery, disease progression, and how cells respond to stress,” said Ding.
Worm Cells Paint a Different Picture
To explore how particles move within multicellular organisms, the research team, co-led by Daniel Starr and G.W. Gant Luxton, utilized protein structures known as Genetically Encoded Multimeric Nanoparticles (GEMs). These GEMs are based on naturally occurring proteins that self-assemble into particles approximately 40 nanometers in diameter, comparable to the size of a ribosome. Their surfaces are coated with fluorescent tags, enabling their movements to be tracked under a microscope at a rapid pace.
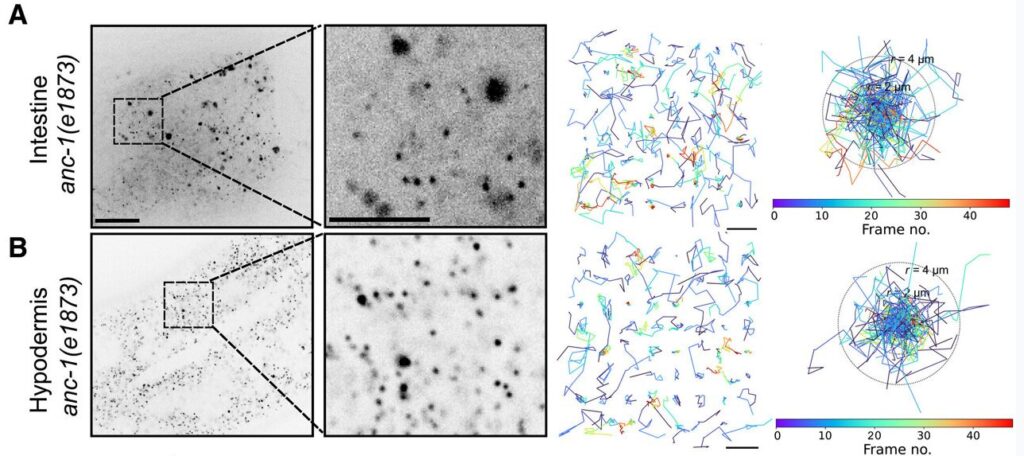
The researchers inserted DNA instructions for GEMs into the genome of Caenorhabditis elegans, a transparent-skinned microscopic nematode. The worms developed normally, but their intestinal and skin cells produced thousands of fluorescently tagged GEMs. Through time-lapse microscopy, the team observed that GEMs moved around 50 times more slowly in C. elegans cells than in cultured mammalian or yeast cells. Moreover, the GEMs were often restricted to certain areas, suggesting a compartmentalized cellular environment.
“When we first noticed that the worm cells were very constrained, we thought it was a mistake, because this is completely different from what is seen in yeast or mammalian tissue culture cells,” Ding noted.
Understanding Cellular Compartmentalization
The researchers sought to understand the mechanisms behind this compartmentalization. They focused on ANC-1, a large protein acting as a scaffold to maintain cellular architecture. Disrupting ANC-1 production did not reduce crowding, but it did allow GEMs to move more freely within the cytoplasm.
Cytoplasmic crowding was instead influenced by ribosome concentration, the same structures that regulate crowding in yeast and mammalian cells. When both ANC-1 and ribosome production were disrupted, GEM movement became significantly faster and less restricted.
“This shows that cells use two complementary systems to control particle mobility,” said Luxton. “The ribosomes are acting like packing peanuts in a box, and the boxes themselves may be the ANC-1 protein complexes.”
The Next Frontier: Biophysics in Living Organisms
Developing the GEM system for use in worms was a challenging process that took years to perfect. Starr emphasized the importance of studying cells in living organisms, noting that the physical environment of tissue-cultured cells differs greatly from that in actual organisms.
With this system in place, the team is eager to explore other cell types within the worms, such as neurons, to study how cytoplasmic properties change during aging and neurodegeneration. They also plan to extend their research to more complex organisms, beginning with zebrafish.
“This is the next frontier,” said Luxton. “We’re trying to figure out how the physical properties of cells contribute to health and disease, and the only way we can do this is by looking at tissues in living organisms at the intersection of developmental genetics and cellular biophysics.”
The implications of this research are vast, potentially influencing fields ranging from drug delivery to understanding disease progression. By delving deeper into the biophysics of living organisms, scientists hope to unlock new pathways for medical advancements and a better understanding of cellular dynamics.