In a groundbreaking development for the brewing industry, a new method utilizing Raman spectroscopy has been introduced to track beer fermentation at the cellular level. This innovative approach, detailed in a recent study published in Bioresource Technology, promises to provide rapid, label-free readings from individual yeast cells, offering insights previously masked by traditional bulk testing methods.
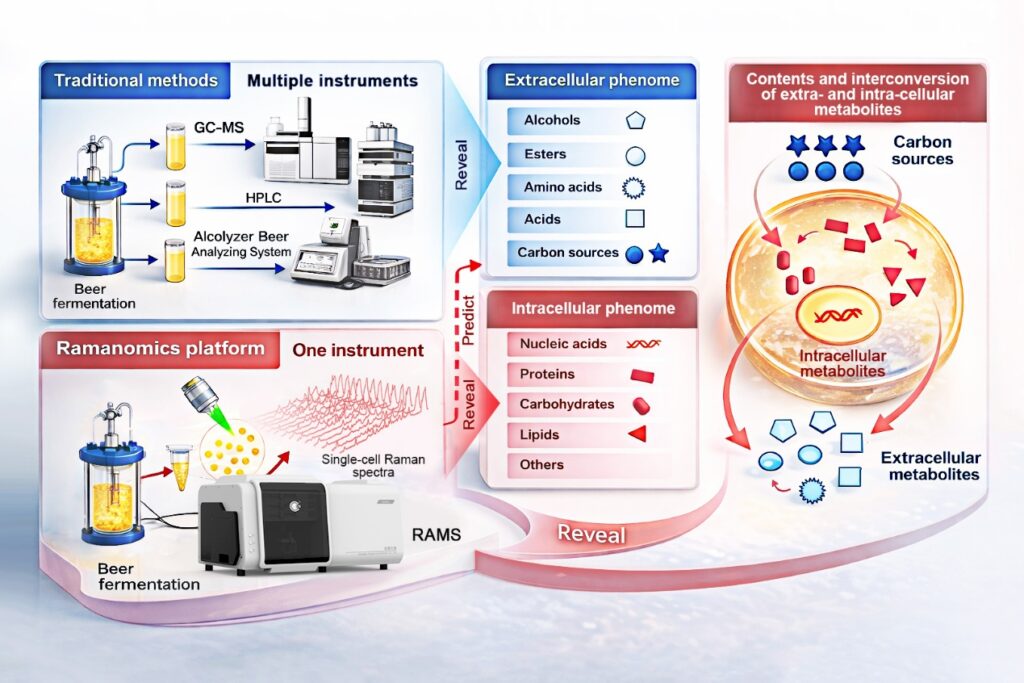
Traditionally, breweries have relied on chromatography-based assays to monitor fermentation, quantifying alcohols, esters, acids, and residual sugars. While these tests are reliable, they are often time-consuming and only provide batch-average results. The new method, developed by scientists from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences, in collaboration with external partners, introduces a novel workflow termed “process ramanomics,” which leverages spontaneous single-cell Raman spectroscopy.
Revolutionizing Fermentation Monitoring
The research team validated their approach by tracking an industrial beer fermentation process using the lager yeast Saccharomyces pastorianus. Over an eight-day period, they sampled a single production batch, collecting high-throughput Raman spectra from individual cells, known as “ramanomes.” These unique molecular fingerprints were then matched to conventional lab measurements of 43 extracellular phenotypes in the fermentation medium.
Through multivariate regression analysis, the team discovered that ramanomes could accurately predict 19 extracellular phenotypes. This included four higher alcohols, four esters, four amino acids, two organic acids, four mono- and disaccharide substrates, and the alcohol-to-ester ratio—a key indicator of beer flavor balance. In practical terms, this means that a single, rapid cellular analysis can now replace multiple time-intensive chemical assays, without sacrificing the detailed insights provided by single-cell resolution.
Unveiling Cellular Heterogeneity
One of the most significant findings of the study is the ability to track phenotypic heterogeneity over time. Different metabolite classes displayed distinct heterogeneity trajectories, with higher heterogeneity often accompanying lower metabolite levels. This suggests that cell dispersion could serve as a useful indicator of process-state.
To delve deeper into the biological mechanisms at play, the researchers developed a new analytical tool called Intra-Ramanome Correlation Analysis (IRCA). This method extracts correlation networks that link intracellular Raman spectral features to extracellular metabolites and substrates. The IRCA-based analysis identified carbohydrates as the most dynamically changing intracellular component pool and revealed that protein-associated Raman signals are closely linked to alcohol and ester production in the early stages of fermentation.
“Instead of waiting for tank chemistry to drift before we notice it, we can now read the cells directly—and infer multiple process outputs from their metabolic fingerprints,” said Prof. XU Jian, co-corresponding author of the study.
Implications for the Brewing Industry
This development comes at a time when the brewing industry is increasingly seeking ways to enhance efficiency and product quality. The ability to monitor fermentation at the cellular level not only accelerates the process but also provides brewers with a more detailed understanding of the fermentation dynamics. This could lead to more consistent product quality and potentially new beer flavors.
Moreover, the insights gained from this method could extend beyond brewing. The principles of “process ramanomics” may be applicable to other fermentation-based industries, such as biofuel production and pharmaceuticals, where understanding cellular behavior is crucial.
As the brewing industry continues to innovate, the introduction of Raman spectroscopy for fermentation monitoring represents a significant leap forward. By providing a fast, label-free window into brewing progress with single-cell resolution, this method could redefine how brewers approach fermentation monitoring.
With further research and development, this technology could become a staple in breweries worldwide, offering unprecedented control over the fermentation process and paving the way for new advancements in brewing science.