In a groundbreaking development, a team of Chinese scientists has unveiled a high-performance iron-based catalyst for proton exchange membrane fuel cells (PEMFCs), potentially reducing the reliance on expensive and scarce platinum. This advancement, published in the prestigious journal Nature, could revolutionize the clean energy sector by making hydrogen fuel cells more accessible and sustainable.
PEMFCs, often dubbed “hydrogen power banks,” are celebrated for their high efficiency, rapid start-up, and zero emissions, making them ideal for applications in transportation, portable electronics, and stationary power generation. However, their widespread adoption has been hindered by the dependency on platinum, a costly and limited resource. The new iron catalyst, described with an “inner activation, outer protection” design, promises record efficiency and long-term durability, offering a viable alternative to platinum.
Breaking New Ground in Catalyst Design
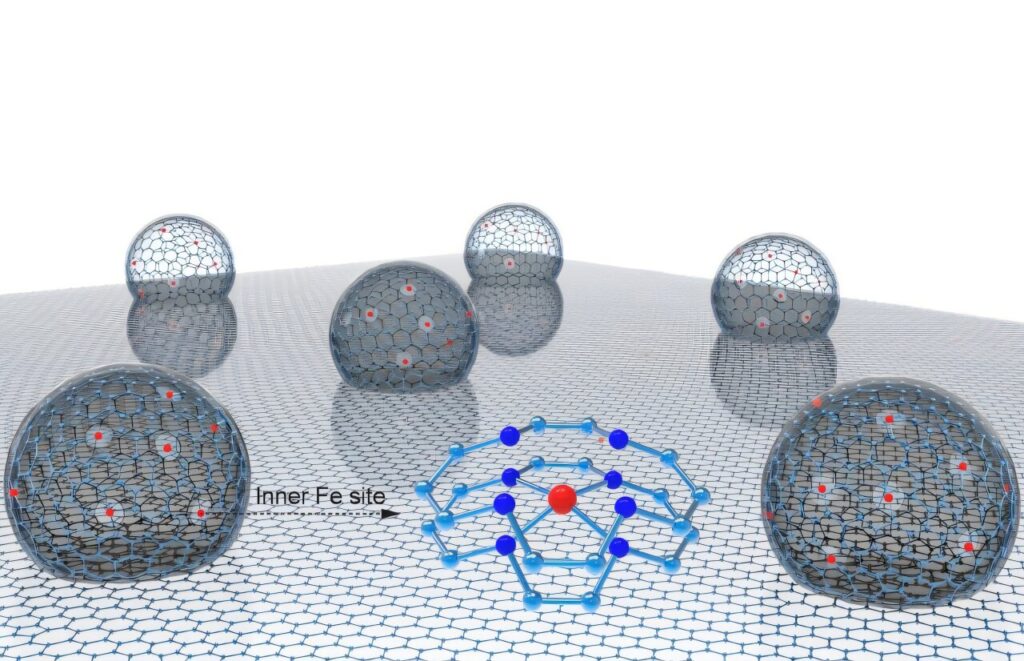
The innovative catalyst, known as CS Fe/N-C, was developed by a research team led by Prof. WANG Dan from Shenzhen University and Prof. Zhang Suojiang from the Institute of Process Engineering of the Chinese Academy of Sciences. The catalyst features a unique nanoconfined hollow multishelled structure (HoMS), with each nano hollow particle measuring approximately 10 nm × 4 nm. These particles contain multiple shells where iron atoms are densely concentrated on the inner layers.
This structural innovation addresses several challenges faced by traditional Fe/N-C catalysts, which typically rely on the outer surface of graphene or carbon supports. Such designs limit the exposure of active sites and are prone to performance degradation due to strong oxygen intermediate binding and vulnerability to oxidative environments.
Scientific Insights and Performance Metrics
Synchrotron X-ray absorption spectroscopy revealed that the inner Fe atoms predominantly exhibit a +2 oxidation state and an FeN4C10 coordination structure. Mössbauer spectroscopy further confirmed that 57.9% of the Fe sites are in a catalytically active low-spin D1 state. Theoretical calculations highlighted that the nitrogen-doped carbon outer shell with Fe vacancies induces significant electrostatic repulsion, weakening the binding strength of oxygenated intermediates and enhancing catalytic performance.
The catalyst achieved an oxygen reduction overpotential as low as 0.34 V, outperforming traditional planar structures. It delivered a record power density of 0.75 W cm-2 under 1.0 bar H2-air with 86% activity retention after more than 300 hours of continuous operation.
Implications for the Future of Clean Energy
The development of this iron-based catalyst marks a significant step forward in the quest for sustainable energy solutions. By reducing the dependency on platinum, this innovation could lower the cost of PEMFCs, making them more economically viable for widespread use. This advancement aligns with global efforts to transition to cleaner energy sources and reduce carbon emissions.
According to the researchers, the “inner activation, outer protection” design not only improves the catalyst’s activity and stability but also provides a new paradigm for developing high-performance catalysts for next-generation electrocatalysts. The graphitized outer N-C layer effectively weakens the binding strength of oxygenated intermediates and suppresses hydroxyl radical generation, thereby enhancing both activity and stability.
Looking Ahead: The Path to Commercialization
The successful implementation of this iron catalyst in PEMFCs could pave the way for broader adoption of hydrogen fuel cells across various sectors. As the world moves towards a more sustainable future, innovations like the CS Fe/N-C catalyst will play a crucial role in shaping the energy landscape.
Further research and development are necessary to scale up production and integrate this technology into existing systems. The potential for this catalyst to replace platinum in fuel cells represents a significant opportunity for reducing costs and increasing the sustainability of hydrogen energy solutions.
For those interested in the latest advancements in science and technology, subscribing to platforms like Phys.org can provide daily insights into breakthroughs and innovations that are shaping our world.