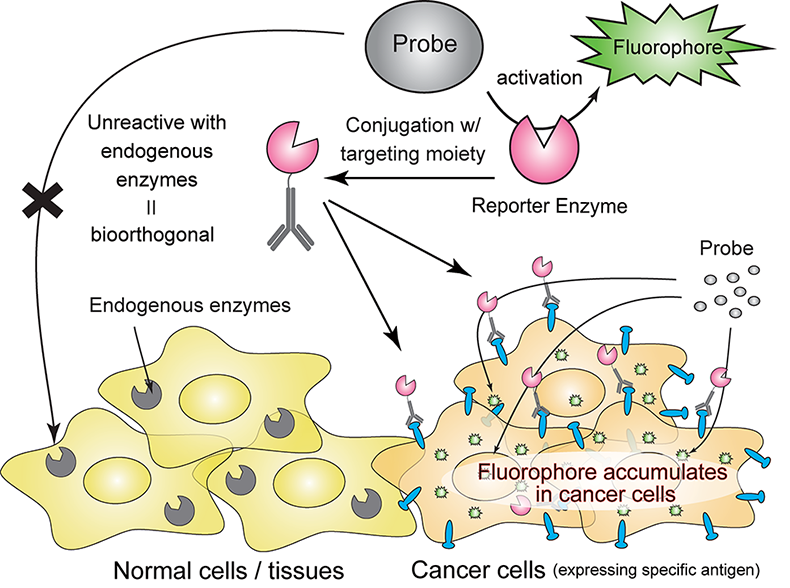
Scientists at Mass General Brigham have unveiled a groundbreaking new tool named LUCAS (Luminescence CAscade-based Sensor) that promises to transform virus detection. Unlike traditional tests, LUCAS is fast, portable, and boasts remarkable accuracy. Utilizing an enzyme that emits a glow akin to fireflies, it enables the detection of virus particles in complex samples such as blood or saliva.
The team’s study, published in Nature Biomedical Engineering, reveals that LUCAS produces signals 500 times brighter and eight times longer-lasting than conventional tests. This enhanced luminescence allows for quicker and more reliable detection of viruses like HIV and COVID-19.
How the Glow Works
Most rapid tests depend on color changes or chemical reactions, often compromising accuracy due to fading fluorescent signals and background noise. LUCAS circumvents these issues by employing bioluminescence, using luciferase—the enzyme responsible for the glow in fireflies. When luciferase interacts with luciferin, it emits light.
Previous tests faced challenges with quickly fading light. LUCAS overcomes this by incorporating beta-galactosidase, an enzyme that gradually releases luciferin, sustaining the reaction and maintaining the glow. This results in a stable, clear signal, significantly simplifying virus detection.
Tests demonstrate that LUCAS generates 515 times more light than non-LUCAS systems, retaining 96% of its initial intensity even after an hour.
Speed, Accuracy, and Simplicity
The research team evaluated LUCAS on over 300 samples, including HIV, hepatitis B and C, and COVID-19, sourced from swabs or blood draws. In every instance, LUCAS delivered results in under 23 minutes, with an average accuracy exceeding 94% across all viruses tested.
This performance is crucial, as swift, reliable results enable faster medical decisions. For those in remote areas, LUCAS offers high-quality testing without the need for extensive training or handling. The device operates independently, without requiring specialized lab equipment or even electricity, making it suitable for hospitals and field clinics alike.
Untrained users achieved results nearly identical to those of trained technicians, highlighting the device’s user-friendly nature.
Why LUCAS Outshines Others
Traditional methods often linked luciferase enzymes directly to antibodies, leading to instability. By the second day, signals would diminish by 77%, disappearing entirely by the third day. LUCAS innovates by attaching beta-galactosidase to antibodies, maintaining 98% of its strength after four days.
Researchers confirmed the longevity of the light by placing enzymes in test plates and monitoring the glow. LUCAS demonstrated robustness, losing less than 4% of its signal after an hour, whereas non-LUCAS systems lost over 25%.
Additionally, the researchers fine-tuned the brightness by adjusting enzyme quantities, enhancing reaction speed and overall brightness. LUCAS requires over 500 times less enzyme than older systems, delivering stronger results with fewer resources.
Designed for the Future
Developing LUCAS involved meticulously preparing its components. Researchers crafted antibodies to bind viruses and combined them with magnetic beads for effective virus particle extraction. They verified each component’s functionality through color tests and chemical analyses.
To ensure real-world applicability, the team tested LUCAS with virus-spiked samples, confirming its ability to detect low infection levels. Whether detecting HIV, hepatitis B, hepatitis C, or SARS-CoV-2, LUCAS consistently performed well.
A crucial aspect of LUCAS’s design is its adaptability to emerging needs. As new viruses or disease markers arise, LUCAS can be modified to detect them, potentially aiding in early detection of conditions like Alzheimer’s disease.
Dr. Sungwan Kim, a postdoctoral researcher at Brigham and Women’s Hospital, stated, “We want to make early detection easier than it’s ever been and push personalized care into a new era.”
A Tool With Global Impact
LUCAS represents a paradigm shift in diagnostics, addressing the urgent need for simple, accurate, and affordable tools. As diseases spread rapidly, many regions lack access to large labs or costly equipment. This technology can bridge that gap, empowering communities to manage their health more independently.
By reducing testing costs and time, LUCAS also supports quicker public health responses. While further testing and development are necessary, its initial results are promising. LUCAS exemplifies how integrating biology and engineering can yield intelligent, effective, and widely applicable tools, potentially setting a new standard in medical diagnostics.