Mitochondrial dysfunction is a critical factor in a range of chronic diseases and cancers, including neurodegenerative disorders and metabolic syndromes. The ability to gently extract a single mitochondrion from within a living cell, without causing damage or relying on fluorescent markers, has long posed a significant challenge for scientists. However, a groundbreaking development from The Hong Kong University of Science and Technology (HKUST) promises to change that narrative.
A team led by Prof. Richard GU Hongri, Assistant Professor in the Division of Integrative Systems and Design at HKUST, has unveiled an automated robotic nanoprobe. This innovative device can navigate within living cells, sense metabolic signals in real-time, and extract individual mitochondria for analysis—all without the need for fluorescent labeling. It stands as the world’s first cell-manipulation nanoprobe that integrates both sensors and actuators at its tip, enabling autonomous navigation inside live cells. This breakthrough holds immense potential for advancing future treatment strategies for chronic diseases and cancer.
From Seeing to Sensing
The mitochondrion, though not much larger than a bacterium, plays a crucial role in sustaining life by carrying out essential chemical processes within every living cell. Traditional intracellular “microsurgery” techniques have heavily relied on manual operations and fluorescent signals, which involve tagging the target, illuminating the sample, and tracking the glow. However, these methods can lead to cell damage due to intense light exposure and interference from fluorescent labels.
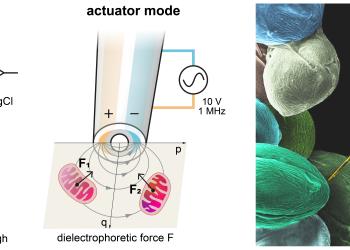
To overcome these limitations, the HKUST research team adopted a novel approach. Instead of attempting to visualize mitochondria, they developed a method to sense them. At the tip of the glass-fine nanoprobe are two nanoelectrodes that detect fleeting surges of reactive oxygen and nitrogen species (ROS/RNS), which are by-products of mitochondrial metabolism. Combined with an automated platform, the tip can track these signals in real-time within living cells.
“Once the signal exceeds a defined threshold, the same tip switches function: tiny dielectrophoretic ‘nanotweezers’ generate a non-uniform electric field that captures a nearby mitochondrion within approximately a hundred nanometers, enabling its extraction at a submicrometric scale with minimal disturbance.”
The key to this approach is colocalization: the sensor and actuator share the very same nanoscale point of action—where the signal is measured is precisely where the organelle is extracted.
Revolutionizing Cell Manipulation with Precision
Equally significant is the process that occurs outside the cell. The team has integrated the nanoprobe into a robotic workflow that standardizes and records each step: approaching the target cell, detecting the cell surface, piercing the cell membrane, tracking electrochemical currents, engaging the dielectrophoretic trap, and safely withdrawing. This procedure reduces invasiveness and enables repeated sampling of the same cell, providing a clearer and more standardized operating workflow.
Ensuring Mitochondrial Functionality and Health
To confirm the presence of extracted mitochondria, quantitative PCR was performed to verify the mitochondrial genetic content. Notably, when transplanted into recipient cells, the imported mitochondria fused with the host network and underwent fission, demonstrating hallmark behaviors of healthy organelles. In other words, the extracted mitochondria can not only return to the cell but also remain functional.
Prof. Gu stated, “Researchers can now sample mitochondria from single living cells without the confounding effects of fluorescent labels. These samples can then be combined with genomics or biochemical assays, providing new insights for minimally invasive surgical research on mitochondrial dysfunction diseases, including neurodegenerative diseases and metabolic syndrome. The system also enables organelle transplantation, advancing the long-imagined ability of assembling ‘designer’ cells from living components.”
A Platform for the Future
Since metabolic or ionic signatures can guide the probe to other organelles, and since the dielectrophoretic traps can be tuned and the robotic protocol retrained, this technology is highly versatile and applicable for extracting mitochondria from various organelles. Looking ahead, the team plans to expand the library of label-free targets, improve probe efficiency, and integrate post-extraction analytics. This initial demonstration marks a more standardized operating procedure for single-cell “microsurgery,” paving the way for transformative advancements in cellular research and therapeutic applications.
The announcement comes as the scientific community continues to explore innovative methods for understanding and treating complex diseases. As this technology evolves, it could significantly impact the field of biomedicine, offering new pathways for research and treatment that were previously unimaginable.





