Bacteria fill your body by the trillions, especially in your gut, where they outnumber your human cells. These tiny organisms, part of what’s known as your microbiome, play crucial roles in digestion, vitamin production, and even influencing your mood and immune system. However, the intricate workings of this system remain largely a mystery.
Why do certain bacteria persist while others fade away? What makes some people’s gut microbes more beneficial than others? Could understanding these mechanisms help prevent or treat diseases? A research team at the California NanoSystems Institute at UCLA has uncovered a new clue that might answer these questions.
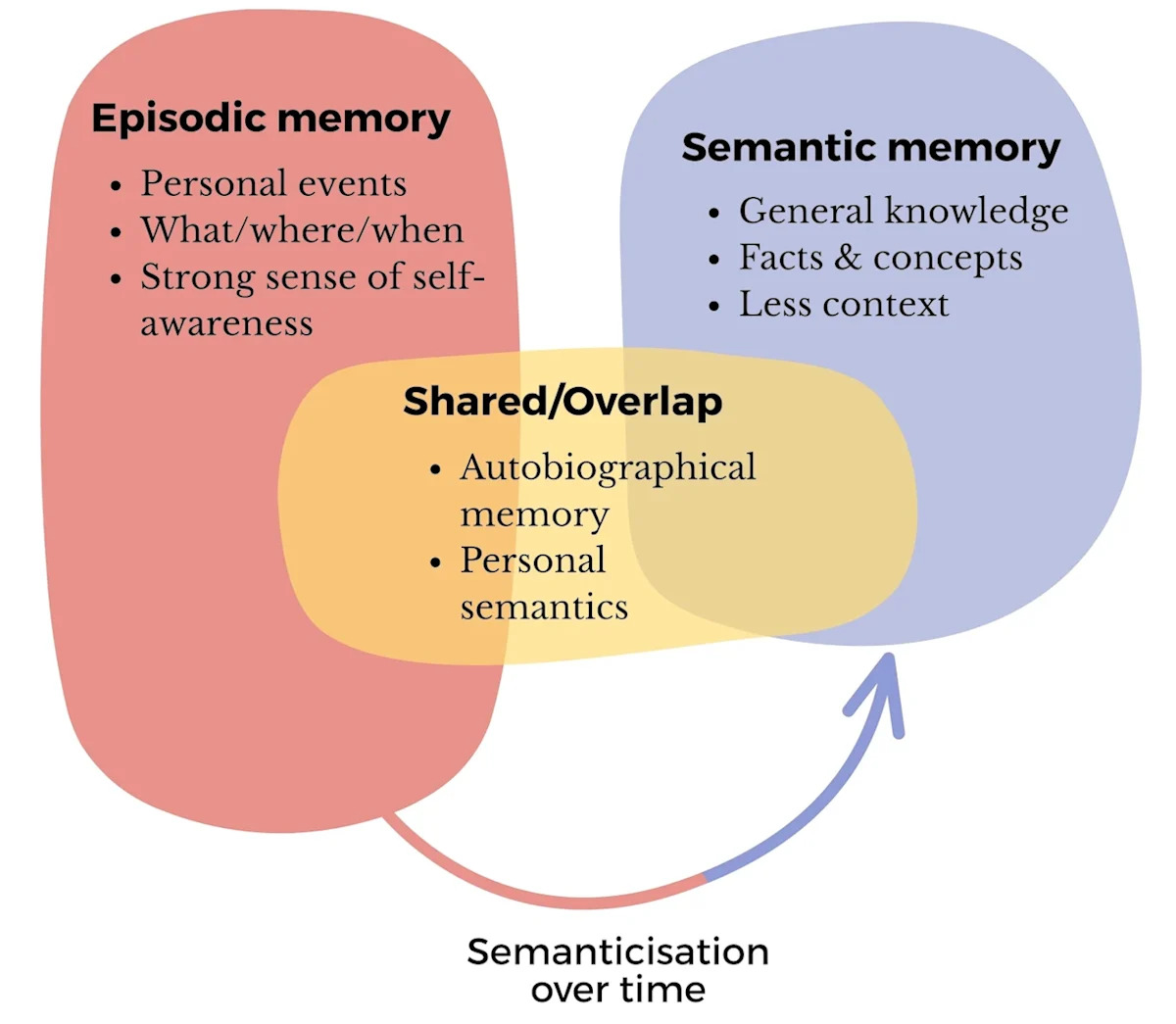
Decoding Bacterial Adaptation: The Role of DGRs
It turns out that gut microbes have a special trick up their sleeve. Some bacteria possess genetic elements known as diversity-generating retroelements, or DGRs. These elements act like mini mutation machines, altering specific genes repeatedly, allowing microbes to adapt to new conditions more swiftly than through traditional evolution.
While DGRs are not new to science, this is the first time researchers have examined their role in the gut. The study focused on bacteria commonly found in healthy human intestines, discovering that approximately one-quarter of the DGRs targeted genes responsible for bacterial adhesion—an essential step in colony formation. These genes control structures called pili, tiny hairlike projections that function like Velcro to help bacteria adhere to surfaces.
In gut bacteria, DGRs continually modify the genes for pili-binding proteins, likely enhancing the microbes’ survival chances in different guts or new environments.
Horizontal Gene Transfer: Spreading Adaptability
The study reveals that DGRs not only aid bacterial adaptation but also spread through horizontal gene transfer. This process allows DGRs to jump from one bacterial strain to another. In studies involving mothers and their infants, researchers found that certain DGRs are inherited during the first year of life. This is significant because the early microbiome—the bacterial community forming in the gut during infancy—plays a vital role in shaping the immune system.
“The developing microbiome is connected to our developing immune system, and that primes us for the rest of our lives,” said Ben Macadangdang, assistant professor of pediatrics at UCLA. “When the microbiome is disrupted, we see higher rates of chronic disease later in life. This presents a strong opportunity to engineer the infant gut microbiome to prevent these risks.”
The Implications of a Dynamic Microbiome
Scientists have long known that a disrupted microbiome is linked to numerous health issues, including inflammatory bowel disease, Crohn’s disease, colon cancer, metabolic syndrome, and even mental health conditions like anxiety, depression, and autism. In children, an increase in harmful gut bacteria early in life is associated with a higher risk of autoimmune diseases later on.
This study adds another piece to the puzzle, demonstrating that DGRs are integral to how gut bacteria evolve, persist, and influence our biology from infancy through adulthood. The DGR system was first discovered in the lab of Jeff F. Miller, director of CNSI and a professor of microbiology at UCLA.
“One of the real mysteries in the microbiome is exactly how bacteria colonize us,” said Miller. “It’s a highly dynamic system intimately connected with human physiology, and this knowledge about DGRs could one day be applied for engineering beneficial microbiomes that promote good health.”
Miller also highlighted the efficiency of DGRs compared to similar systems in the human body. While immune cells can produce a vast variety of antibodies, they do so once per cell. In contrast, DGRs can generate mutations repeatedly within the same cell, creating far more diversity.
To illustrate, if each unique antibody in your immune system were a grain of sand, it would take less than a quarter of 1% of the Empire State Building to hold them all. For DGRs? You’d need the equivalent of 270 million Empire State Buildings.
The Future of Gut Microbiome Research
In their study, the researchers examined gut bacteria from the Bacteroides group, a common resident of the human gut, identifying more than 1,100 different DGRs. On average, each bacterial strain carried at least one, with some having up to five. The team focused on those affecting pili genes—the sticky structures aiding bacterial adhesion.
The DGRs’ role was to continuously remix the proteins that help these pili function. This adaptability likely explains why these bacteria thrive in diverse human hosts.
“We think DGRs allow the bacteria to rapidly change what their pili can adhere to,” said Macadangdang. “A bacterium may be optimized for one person’s gut, but if it goes out and tries to colonize someone else, it encounters a very different environment. Finding something new to bind to gives the bacteria an advantage, and we think that’s why we see so many DGRs within the microbiome.”
The research team is just beginning to explore the potential of DGRs. They aim to further investigate how these elements function in both healthy and unhealthy guts, with the hope that this knowledge could lead to innovative ways to engineer the microbiome. This could involve using DGRs themselves to treat disease, enhance immunity, or tailor gut bacteria for improved health outcomes.
“We’re at this really early stage,” Miller said. “There are so many questions that this raises, we’re just realizing how much we don’t know about DGRs in the microbiome, or what exploiting them for applications could yield. I’ve never been more excited about what’s going to come next.”
The full study was published in the journal Science.