The most dangerous animal in the world just got easier to study—and perhaps defeat one day. Researchers from Rockefeller University’s Laboratory of Neurogenetics and Behavior, in collaboration with mosquito experts worldwide, have unveiled the first-ever cellular atlas of the Aedes aegypti mosquito. This species transmits more diseases than any other of its kind. The Mosquito Cell Atlas provides cellular-level resolution of gene expression in every mosquito tissue, from the antennae to the legs, and is freely available to researchers and the public. The atlas was recently published in the journal Cell.
“This is a comprehensive snapshot of what every cell in the mosquito is doing as far as expressing genes,” said Leslie Vosshall, lab head and a veteran researcher of the Aedes aegypti, also known as the yellow fever mosquito. “It’s a real achievement because we profiled so many different types of tissues in both males and females.”
Revolutionary Insights into Mosquito Biology
The atlas has already yielded new insights into the genetic secrets of Aedes aegypti, including novel cell types and subtle differences—and unexpected similarities—between male and female mosquitoes. Notably, it reveals the dramatic changes in genetic expression that occur in the female mosquito brain after a blood feeding.
Senior author Nadav Shai, a senior scientist at both Vosshall’s lab and the Howard Hughes Medical Institute, anticipates that the atlas will be a catalyst for new discoveries. “We believe this enormous data set will really move mosquito biology forward,” he stated. “It’s a great tool for vector biologists to take whatever interests them and just run with their own line of research.”
Organ by Organ
In recent years, scientists have used single-cell sequencing to identify cell types and illuminate gene expression patterns in model organisms such as the fruit fly, nematode, and mouse, resulting in a single-cell atlas for each species. Mosquito researchers have been following suit, but in a piecemeal manner—organ by organ, tissue by tissue, across different studies. Some of this prior work was conducted by Vosshall’s team and members of the Aedes aegypti Mosquito Cell Atlas Consortium, a global collaboration of scientists assembled for this project.
Most prior studies had focused on female mosquitoes, leaving males largely unexplored. “Both females and males feed on nectar in their day-to-day lives, but females need blood for protein to develop their eggs and produce a new generation of mosquitoes,” explained first author Olivia Goldman. “Because the female is the one spreading all the pathogens, there is an enormous bias toward studying the biology of the female, with very little information about the male,” Vosshall added. “So we wanted to be inclusive and fill in the gap.”
Advanced Techniques and Surprising Discoveries
To that end, the team employed single-nucleus RNA sequencing (snRNA-seq), which excels at capturing the biology of all insect cell types compared to single-cell approaches. This led to a large dataset of more than 367,000 nuclei from 19 types of mosquito tissues selected across five biological themes: major body segments, sensation and host seeking, viral infection, reproduction, and the central nervous system.
Tasting Sweetness with Their Legs
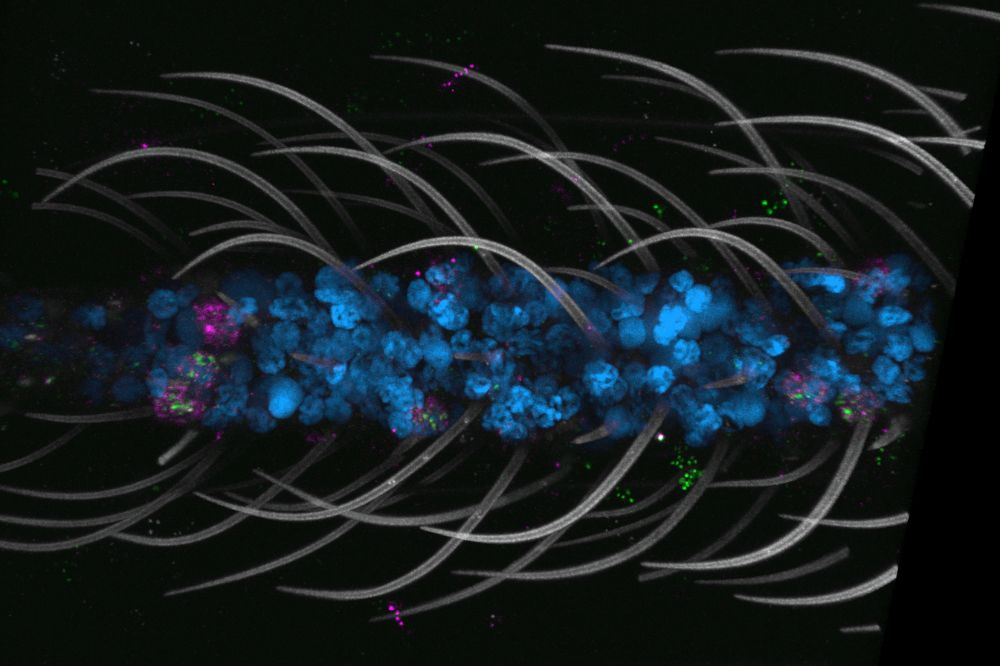
Among the most striking findings was the pervasiveness of polymodal sensory neurons—supercharged cells that can detect a wide variety of environmental cues, including temperature and taste. Previous research from the Vosshall lab had found these neurons in the antenna and maxillary palps, but they are now known to be widespread, including in the nose, tongue, and legs.
“Just like the antennae and maxillary palps, the legs and mouth parts have really powerful tools for sensing the world,” Shai noted. “Together they enable mosquitoes to be really good at what they do—seek hosts, feed on them, and reproduce.” These multifunctional chemoreceptors allow mosquitoes to detect sweetness and fresh water, among other things.
“Being able to taste sweetness with their legs may be useful for detecting sugars, which both females and males need to live,” Shai explained. “But it’s just one part of a combination of tastes that clues them into what’s around them—a human to bite, a flower for a sugar source, or a good water source to lay eggs.”
Brain Changes That Accompany Behavior Shifts
After feeding, a female mosquito loses all interest in humans and other hosts, focusing instead on developing and laying eggs. “How does this incredibly strong drive to bite people get turned off?” Vosshall pondered.
To investigate, the team examined the gene expression of female mosquito brains at 3, 12, 24, and 48 hours after a blood feeding. They found dramatic changes in gene expression at all time points, peaking after the first few hours and gradually abating. Most early expression changes involved genes being upregulated, while later periods showed a mix of both up- and downregulation.
“The glia are completely rewired during this time when the females lose interest in people,” Vosshall said. “That was a big surprise,” Shai added. “It’s evidence that glia are super important for not only supporting brain cells and function but also are physiologically relevant to behavior.”
Implications for Future Research
Another illuminating finding is that despite the documented morphological and behavioral differences between female and male mosquitoes, their cellular makeup is largely identical, aside from small clusters of sex-specific cells and reproductive organs. “We were kind of expecting it to be a tale of two genomes, but that’s not what we found,” Vosshall stated.
“In general, most cells look the same, and the transcripts they express are similar,” Shai noted. “However, that doesn’t mean that the regulation and level of expression are the same, and those probably drive the differences. Another factor could be how the different gene expressions work together to create new functions.”
The Vosshall lab plans to mine the mosquito single-cell atlas further to explore behaviors such as host seeking and environmental sensing through Aedes aegypti’s remarkable, widely dispersed set of multifunctional sensory neurons. “Different people in the lab are going to take it to different places,” Shai said.
They hope researchers worldwide will find it equally inspiring. “The sheer size of the dataset opens up many new avenues of research that people couldn’t study before because they didn’t have this tool,” Shai emphasized. “This is a global resource that has been open to everyone since the very inception of the project in 2021, so many people are already using it,” Vosshall added. “We’re excited to see the discoveries that will come from it.”