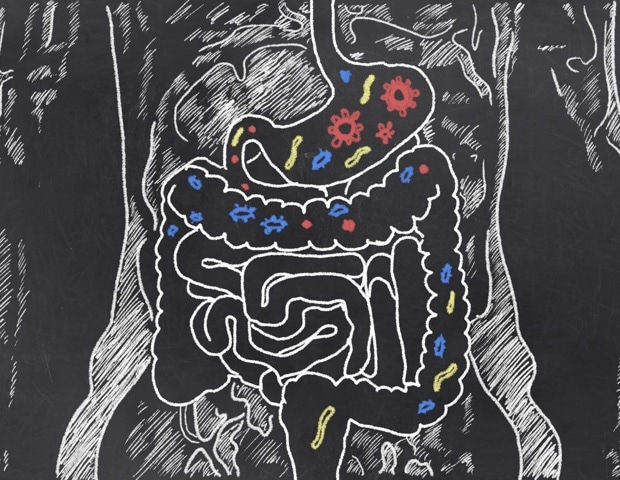
The gut microbiome, a complex ecosystem of trillions of microbes residing in the digestive tract, plays a crucial role in digestion and overall health. While diet and medication have long been known to shape these microbial communities, the extent of genetic influence has remained elusive. A groundbreaking study involving rats, a common model for human gut research, has now revealed that the composition of the gut microbiome is influenced not only by an individual’s genes but also by the genes of those they live with.
This discovery, published on December 18, 2025, in Nature Communications, highlights a novel interaction between genetics and social behavior through the exchange of commensal gut microbes. Conducted by researchers at the University of California San Diego and the Centre for Genomic Regulation in Barcelona, the study offers new insights into how genetics and the microbiome may interact in human diseases.
Unraveling Genetic Links to Gut Microbes
In humans, only two genes have been reliably associated with gut bacteria: the lactase gene, which affects milk-digesting microbes, and the ABO blood-group gene, which influences microbes through mechanisms yet to be understood. Despite the likelihood of more gene-microbe connections, they have been challenging to identify.
To delve deeper into genetic influences on the microbiome, researchers turned to rats, which share many biological characteristics with mammals and can be studied under controlled conditions. “The things that live in their gut are similar but not identical,” explained co-author Abraham Palmer, Ph.D., professor and vice chair for basic research at UC San Diego School of Medicine.
“This was a nice opportunity because the animals are all eating the same food, so we don’t have to worry about genes influencing their microbiome via their food choices, for example. It’s a much simpler system.” – Abraham Palmer, Ph.D.
Key Genetic Discoveries
The study analyzed genetic and microbiome data from 4,000 genetically unique rats across four U.S. facilities. Researchers identified three genetic regions consistently affecting gut bacteria, regardless of environmental differences. The strongest link was between the St6galnac1 gene, which adds sugar molecules to gut mucus, and the abundance of Paraprevotella, a bacterium that feeds on these sugars.
Another region, containing genes that form the protective mucus layer, correlated with Firmicutes bacteria. A third region included the Pip gene, encoding an antibacterial peptide, associated with Muribaculaceae, a bacterial family common in both rodents and humans.
The Social Dynamics of Genes
While genes themselves do not transfer between individuals, microbes can. The study found that certain genes favor specific gut bacteria, which can spread through social contact. “This is the result of genetic influences spilling over to others through social contact,” said senior author Amelie Baud, Ph.D., from the Centre for Genomic Regulation.
“Genes shape the gut microbiome, and we found that it is not just our own genes that matter.” – Amelie Baud, Ph.D.
The study’s size enabled researchers to estimate how much of each rat’s microbiome was influenced by its own genes versus those of its cage-mates. This phenomenon, known as “indirect genetic effects,” is akin to a mother’s genes affecting her offspring’s growth through the environment she provides.
By assigning rats to random social partners, researchers eliminated the confounding factors present in human studies, where social partners are typically self-selected. They discovered that the abundance of some Muribaculaceae species was shaped by genetic effects spreading socially through microbial exchange.
Implications for Human Health
The study’s findings suggest a novel mechanism by which the genetics of one individual can impact an entire social group, altering biological outcomes without changing DNA. This has significant implications for understanding disease risk, as the gut microbiome is increasingly recognized for its role in health.
“Although the details will be different in humans from what we find in rats, the study points the way towards understanding the mechanisms of how host and microbial genes work together to produce complex diseases that the microbiome is involved in, which range from cardiovascular disease to obesity to Alzheimer’s.” – Rob Knight, Ph.D.
As profiling methods improve, more microbes are likely to be identified, expanding our understanding of genetic and microbial interactions. This research could lead to new approaches in predicting and managing diseases by considering both genetic and social factors.
Additional researchers contributing to the study include Denghui Chen, Antonio Gonzalez, Tomasz Kosciolek, and Oksana Polesskaya from UC San Diego; Helene Tonnele from the Centre for Genomic Regulation; and many others from institutions worldwide. The study was partially funded by the National Institutes of Health.