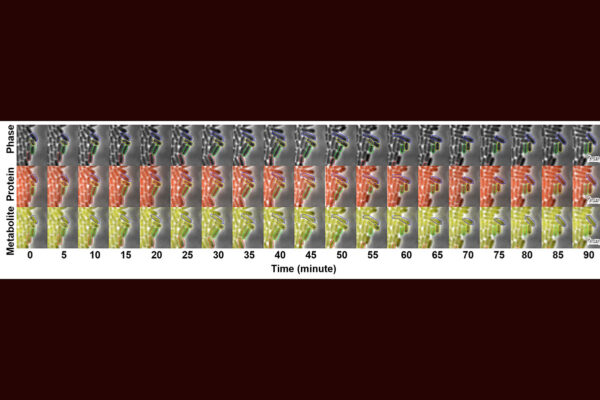
Experts from the University of Nottingham have embarked on a groundbreaking research initiative to explore how genes responsible for disease and antifungal resistance evolve and spread within fungal populations. This vital research is made possible by a £1.2 million grant from the Wellcome Trust, awarded to Professor Paul Dyer, a leading figure in Fungal Biology at the School of Life Sciences. The project also includes collaboration with Dr. Jasmine Ono and researchers from the Universities of Manchester and Pretoria.
The research aims to illuminate the mechanisms by which genes linked to human disease and antifungal resistance proliferate, potentially through sexual and other transfer mechanisms. This understanding is crucial for assessing the risk posed by fungal diseases and informing future health management strategies. Additionally, the study will examine the impact of climate change on the evolution and dissemination of fungal pathogenicity genes.
Understanding the Threat of Fungal Diseases
Fungal diseases such as life-threatening Aspergillosis and Candidiasis, along with tropical Fusarium diseases, are at the forefront of this study. These conditions pose significant risks to human health, and there is growing concern that subpopulations of these fungi may be evolving into new, disease-causing species.
Professor Dyer emphasized the urgency of this research, stating, “Although many fungi are beneficial, there has been a recent rise in threats from fungal diseases to humans, animals, and our plant food crops. This has been compounded by the evolution of resistance to the antifungals that we rely on to control disease.”
Leveraging Advanced Scientific Techniques
The research will utilize cutting-edge developments in genomic, bioinformatic, gene editing, and barcoding analyses. These advanced techniques offer unprecedented opportunities to delve into the genetic intricacies of fungal populations and their potential to evolve pathogenic traits.
“The funding from the Wellcome Trust will allow our team to study the mechanisms of how genes contributing to pathogenicity and drug resistance can evolve and spread through fungal populations, with associated risk, and how this might be impacted by climate change. This is critical to inform fungal disease management and future drug development. We are very grateful to Wellcome for providing us with this opportunity.” – Professor Paul Dyer
The Broader Implications of the Study
This research is not only pivotal for understanding current threats but also for anticipating future challenges posed by fungal diseases. As climate change continues to alter ecosystems worldwide, the potential for fungi to adapt and spread pathogenic genes becomes an increasingly pressing concern.
The project represents a proactive approach to fungal disease management, aiming to provide insights that could guide the development of new antifungal drugs and strategies to mitigate the spread of resistant strains. By understanding how these genes evolve and spread, researchers hope to stay ahead of the curve in combating fungal threats.
Looking Forward: Potential Outcomes and Next Steps
The outcomes of this research could have far-reaching impacts on public health policies and the development of antifungal treatments. As the study progresses, the findings will likely inform both scientific understanding and practical applications in disease prevention and management.
Meanwhile, the collaboration between international research teams underscores the global nature of this challenge and the necessity for a coordinated response. As Professor Dyer and his team delve deeper into their research, the scientific community eagerly anticipates the insights that will emerge, potentially reshaping how we approach fungal diseases in the future.