In recent years, the study of exosomes has emerged as a transformative field in reproductive biology, offering profound insights into cellular communication and oocyte viability. Exosomes, a type of nanoscale extracellular vesicles (EVs), have been recognized for their diverse roles in transporting bioactive molecules such as proteins, microRNAs (miRNAs), and lipids. These vesicles are generally categorized into small vesicles (SEVs), known as exosomes, and larger vesicles (LEVs), known as microvesicles. Understanding the heterogeneity of these vesicles is crucial for deciphering their roles in cell-to-cell signaling, disease progression, and therapeutic applications.
The announcement comes as recent studies have highlighted the role of exosomes in embryo development and oocyte competence during pregnancy. Exosomes originate from the budding of multivesicular bodies (MVBs) in the endosomal system and are released into the extracellular space. Initially considered as mere cellular waste carriers, exosomes are now understood to be rich in bioactive molecules, establishing paracrine communication between cells and playing a key role in physiological and pathological processes.
Exosomes as Biomarkers and Diagnostic Tools
The potential of exosomes as biomarkers is underscored by their ability to reflect the real-time metabolic state of donor cells. This characteristic positions exosomes as valuable tools for monitoring a range of pathologies, including degenerative diseases, neoplastic changes, and metabolic disorders. Their presence in various bodily fluids such as blood, urine, and breast milk enhances their potential as natural diagnostic tools.
Meanwhile, studies have begun to elucidate the physiological significance of exosomes in the reproductive system. Exosomes may be utilized to monitor pathological conditions based on their molecular signatures in reproductive tissues. The capacity of eukaryotic cells to uptake and secrete exosomes into their surrounding microenvironments further emphasizes their ubiquity and biological relevance.
Exosomes in Oocyte Competence and Fertility
Research indicates that exosomes play a crucial role in assessing and modulating oocyte competence, a key factor in achieving successful fertilization and supporting early embryonic development. The quality and quantity of exosomes produced can vary under different physiological and pathological conditions, directly affecting oocyte competence. Understanding these changes can provide insights into the mechanisms underlying oocyte quality and fertility.
In the context of assisted reproductive technologies (ART), analyzing the exosomal content of oocytes or surrounding cells could help identify biomarkers that predict oocyte quality and growth potential. This could lead to improved selection criteria for oocytes used in in vitro fertilization (IVF) procedures, ultimately increasing success rates.
Biogenesis and Isolation Techniques
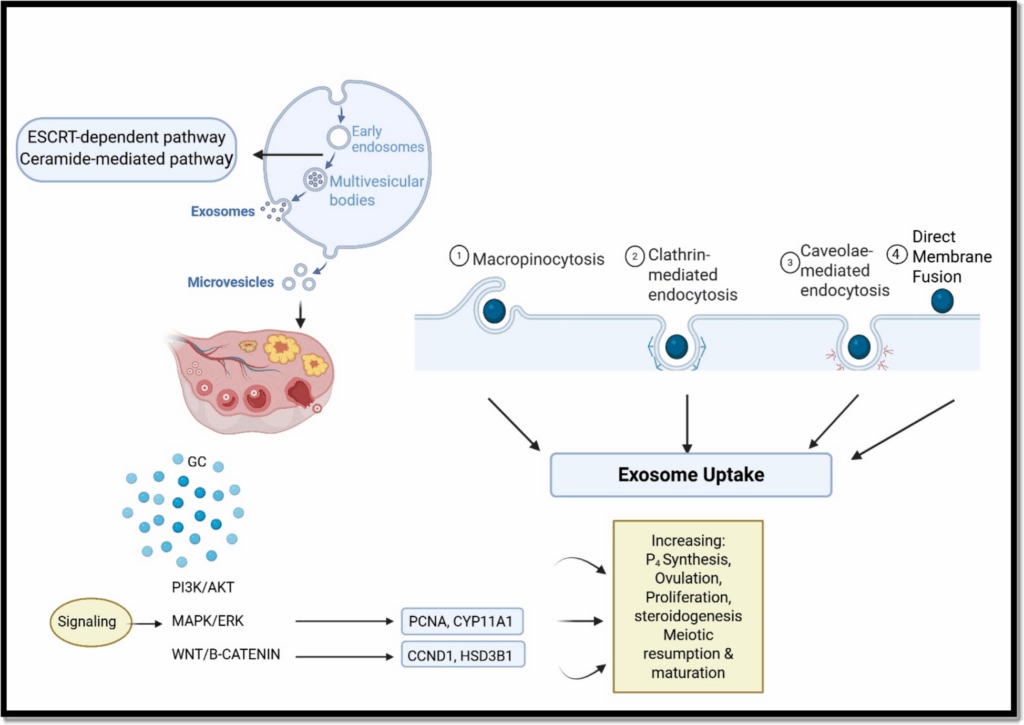
To orchestrate exosome production, an intracellular endosomal system with various effectors exists in host cells. Intraluminal vesicles (ILVs) are generated within multivesicular bodies (MVBs) and can be released into the extracellular matrix as exosomes or directed toward lysosomal degradation. The biogenesis of exosomes involves both ESCRT-dependent and ESCRT-independent pathways, with lipids and proteins playing significant roles in exosome production.
Numerous techniques have been developed for isolating exosomes from cell culture media and biological specimens, each offering unique advantages and limitations. Ultracentrifugation is the most prevalent method, using high-speed rotational forces to separate exosomes based on size and density. Solvent precipitation methods, column chromatography, and immunoaffinity capture are also extensively used, each with its own benefits and drawbacks.
Challenges and Future Directions
Despite the diversity of isolation techniques, understanding how each method influences the physicochemical properties of exosomes remains limited. These parameters directly impact the biological behavior, pharmacokinetics, and therapeutic efficacy of exosomes, especially when used as drug delivery vehicles. Selecting an appropriate isolation method should be tailored to the specific goals of the research or clinical application.
Ongoing research efforts are focused on refining and optimizing isolation techniques to improve yield, enhance purity, and better preserve the native structure and functional integrity of exosomes. Advances in this area are vital for unlocking the full potential of exosomes in various biomedical fields, including the development of exosome-based therapeutics, diagnostics, and regenerative medicine.
Exosomes in Oocyte and Granulosa Cell Interaction
Histological examinations reveal that oocytes are surrounded by granulosa cells (GCs) within follicles. The interaction between oocytes and GCs is crucial for oocyte maturation and competence. Exosomes facilitate this interaction by carrying signaling molecules between cells, influencing oocyte competence and fertility outcomes.
Studies have shown that follicular fluid (FF) exosomes can regulate the proliferation of cumulus GCs and affect oocyte competence in various mammalian species. The exosomal cargo, consisting of proteins, RNAs, and lipids, can alter the function of oocytes, making exosomes a valid theranostic agent in terms of oocyte competence.
Therapeutic Potential and Clinical Implications
Exosomes hold promise as therapeutic agents in reproductive medicine. They can modulate oxidative stress, mitochondrial function, and oocyte viability, offering potential solutions for age-related infertility, metabolic disorders, and assisted reproductive technology challenges. However, challenges such as exosome heterogeneity, variability in isolation methods, and unresolved safety concerns remain barriers to clinical translation.
In conclusion, exosomes are indispensable in reproductive biology, offering insights into oocyte competence and fertility. Their dual role as biomarkers and therapeutic agents holds promise for addressing infertility, ovarian aging, and metabolic disorders. Future research must prioritize standardization of techniques and mechanistic studies to unlock their full potential in reproductive medicine.







