Autoimmune diseases, a group of disorders characterized by the immune system attacking the body’s own tissues, involve a complex interplay of immune dysregulation. Central to these processes are cytokines, soluble proteins that mediate communication between immune cells. These signaling molecules are pivotal in both maintaining immune homeostasis and driving the pathogenesis of diseases such as systemic lupus erythematosus (SLE), psoriasis, rheumatoid arthritis (RA), and inflammatory myopathies.
The critical role of cytokines in autoimmune diseases underscores their dual functionality. While they are essential regulators of immune responses, they can also act as pathogenic drivers. This paradox has led to significant research efforts aimed at understanding cytokine networks and developing targeted therapies.
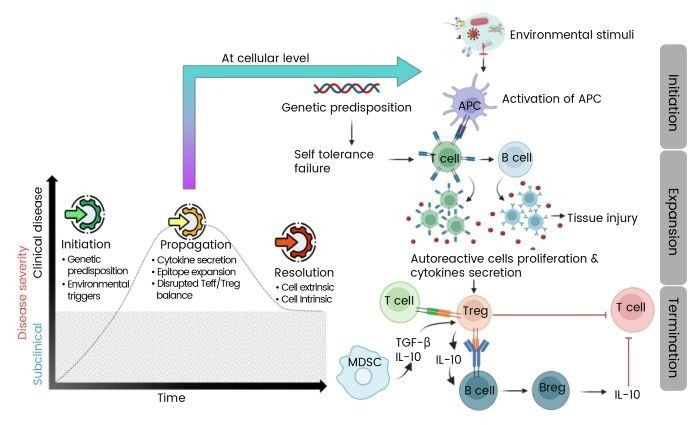
Mechanisms of Autoimmunity
Autoimmunity arises from disruptions in immune signaling pathways, often triggered by genetic predispositions and environmental factors. These disruptions lead to excessive cytokine production, activation of self-reactive lymphocytes, and the release of autoantibodies, collectively damaging normal tissues. However, resolution of autoimmune processes is sometimes possible through the restoration of regulatory mechanisms involving regulatory T cells (Tregs) and regulatory B cells (Bregs).
These regulatory cells produce immunosuppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), which help repair tissue damage and suppress inflammatory pathways. The balance between pro-inflammatory and regulatory cytokines is crucial in determining the progression or resolution of autoimmune diseases.
Cytokine Networks in Autoimmune Pathogenesis
Pro-inflammatory Cytokine Dominance
The pathogenesis of autoimmune diseases is often driven by a hyperactive pro-inflammatory cytokine environment. Cytokines such as IL-6, IL-17, and tumor necrosis factor-alpha (TNF-α) are core mediators in conditions like psoriasis and RA. The IL-1 family, including IL-1β and IL-18, exacerbates tissue damage by activating mechanisms like the NLRP3 inflammasome, implicated in SLE.
Not only do these cytokines drive local inflammation, but they also contribute to systemic manifestations like fatigue and fever via their actions on the hypothalamic-pituitary-adrenal axis.
Regulatory Cytokine Deficiencies
Immunosuppressive cytokines such as IL-10 and TGF-β play protective roles by counterbalancing pro-inflammatory signals. Reduced IL-10 production by Bregs has been correlated with disease flares in SLE, highlighting its protective role. TGF-β maintains peripheral tolerance by suppressing effector T cell proliferation and inducing Treg differentiation.
Dysregulation of these pathways creates a permissive environment for autoimmunity, as seen in RA and multiple sclerosis (MS), where defective TGF-β signaling permits unchecked Th17 activity.
Cytokine Imbalance and Feedback Loops
Autoimmune diseases typically exhibit self-reinforcing cytokine feedback loops. For instance, IL-6 enhances Th17 differentiation while inhibiting Treg development, creating a pathogenic cycle that sustains inflammation. In psoriasis, Th17 survival is perpetuated by IL-23, resulting in the secretion of IL-17 and IL-22, further activating stromal cells and keratinocytes.
Therapeutic Targeting of Cytokine Networks
Biologic Therapies
Biologic therapies have revolutionized the treatment of autoimmune diseases by targeting specific cytokines. Anti-TNF-α agents like adalimumab and infliximab have transformed RA treatment by reducing synovitis and radiographic progression. However, TNF blockade may induce psoriasiform lesions in some patients, underscoring cytokine pleiotropy.
IL-6 receptor antagonists, such as Tocilizumab, have shown efficacy in reducing systemic inflammation in juvenile idiopathic arthritis (JIA) and RA. IL-17/IL-23 axis targeting with agents like Secukinumab and Ustekinumab has achieved rapid skin clearance in psoriasis.
JAK/STAT Inhibition
JAK inhibitors, or jakinibs, block downstream STAT phosphorylation, effectively modulating cytokine signaling. Tofacitinib and baricitinib are approved for RA treatment, working by suppressing pathways like IFN-γ, IL-6, and GM-CSF. These inhibitors also reduce IFN-α signature and ameliorate nephritis in SLE, offering alternatives to broad immunosuppression.
Emerging Therapies
Innovative approaches such as low-dose IL-2 therapies aim to expand Tregs and restore immune tolerance in diseases like type 1 diabetes and SLE. Engineered IL-2 variants with improved specificity have shown promise in preclinical models, though clinical trials report varying efficacy.
miRNA modulation represents another frontier, with miRNAs regulating cytokine production post-transcriptionally. For instance, miR-155 promotes TNF-α and IL-6 in RA synovium, while miR-146a inhibits NF-κB signaling. Antagomirs targeting miR-155 have reduced disease severity in experimental autoimmune encephalomyelitis (EAE).
Conclusion and Future Directions
Pioneering developments in cytokine biology have enabled the identification of disease-specific signatures, facilitating precision therapies targeting key nodes within these networks. Challenges remain, including cytokine pleiotropy, interpatient heterogeneity, and redundancy.
Ongoing research areas include personalized cytokine profiling, microbiome-directed interventions, and engineered biologics with enhanced cell-type specificity.
The integration of mechanistic insights with innovative therapeutics is poised to open up the next frontier in autoimmunity, effectively harnessing the cytokine network to restore immune equilibrium.
Sino Biological provides a range of high-quality cytokine products designed to support targeted research and drug development for autoimmune diseases. The company’s commitment to quality ensures high purity, stability, and bioactivity of its products, supporting advancements in early detection and targeted therapy development.





