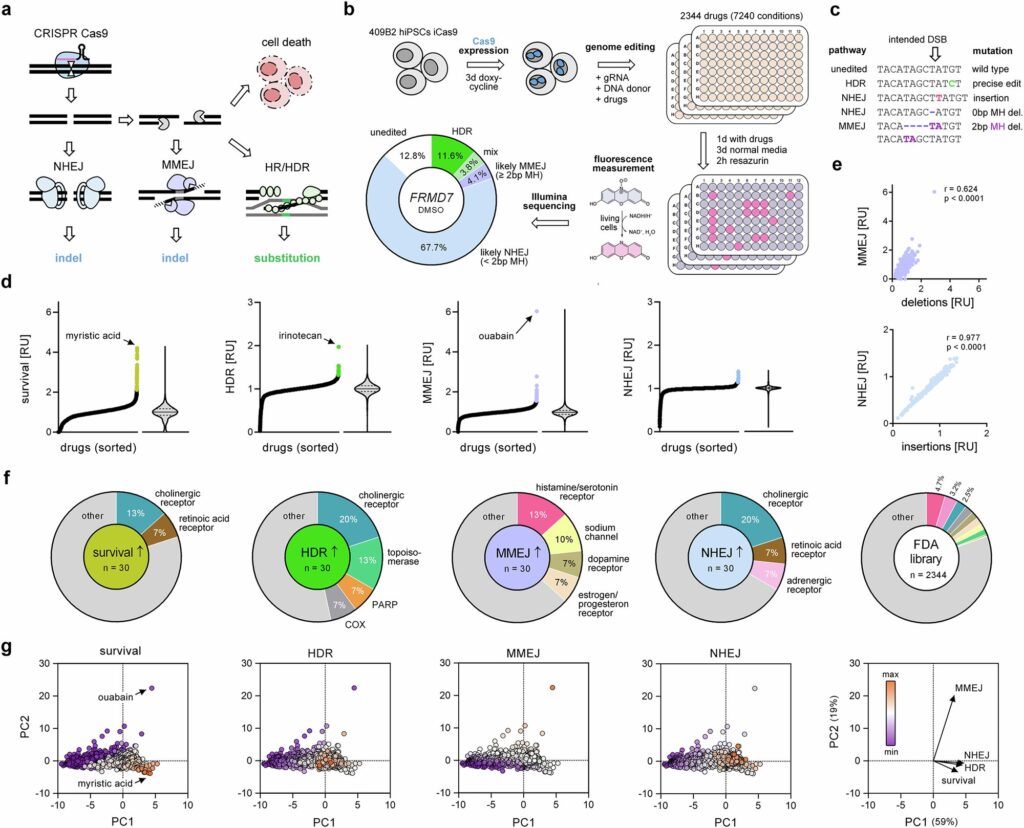
In a groundbreaking study, scientists at the Max Planck Institute for Evolutionary Anthropology in Leipzig have uncovered how over 2,000 clinically approved drugs can influence DNA repair and the outcomes of CRISPR genome editing. This research not only highlights compounds that enhance genome editing but also identifies molecules capable of selectively targeting cancer cells, revealing novel roles in DNA repair for two specific proteins.
The announcement comes as CRISPR-based therapies are poised to revolutionize precision medicine, offering new hope in the treatment of genetic disorders and cancer. Understanding the interaction between common medications and these cutting-edge therapies is crucial as they move from research labs into clinical settings.
How DNA Repair Affects Genome Editing
DNA double-strand breaks are critical lesions that can be repaired through various pathways. Some repair processes are rapid but introduce additional mutations, while others are slower yet allow for precise corrections. These pathways are integral to genome editing, where CRISPR-Cas gene scissors are used to cut DNA at specific genome locations. The cells must then repair the break, often using a DNA template with the desired mutation.
The efficiency of incorporating these mutations largely depends on the repair pathway’s activity, necessitating tools to inhibit competing pathways and enhance desired outcomes. The Max Planck team investigated how FDA-approved drugs affect DNA repair pathway selection, a crucial step as CRISPR therapies enter clinical use.
“Understanding how everyday medicines interact with CRISPR-based treatments will be increasingly important as these therapies enter real-world clinical use,” says Dominik Macak, one of the study’s lead authors.
Drug Interactions and New Discoveries
With the first CRISPR gene therapy approved in the U.S., U.K., and EU in late 2023, patients undergoing such treatments may also be on medications for infections or chronic conditions. These routine drugs can influence cellular processes like DNA repair, potentially affecting therapy efficacy and safety.
The scientists developed a comprehensive atlas depicting how clinically approved drugs impact DNA repair in human cells. Testing over 7,000 drug conditions, they assessed how each compound alters DNA repair post-CRISPR cut.
“We anticipate that this catalog will serve as a valuable resource for clinicians and researchers working in disease modeling, gene therapy, and oncology,” adds co-lead author Philipp Kanis.
Notably, the team identified several pharmaceuticals that influence major repair pathways. The screening data led to the discovery of previously unrecognized drug targets significantly affecting repair outcomes. Among these, two proteins—estrogen receptor 2 (ESR2) and aldehyde oxidase 1 (AOX1)—were found to play novel roles in DNA repair.
Potential Implications for Cancer Treatment
Targeted inhibition of ESR2 can increase the efficiency of precise genome edits by up to fourfold. Meanwhile, drugs inhibiting AOX1 can be used to kill cultured cancer cells lacking one repair pathway—a condition common in many cancer cells.
“Our study identifies several approved medicines as promising candidates for treating cancers with DNA-repair deficiencies, offering potential options beyond current therapies,” says Stephan Riesenberg, senior researcher on the project.
Despite these promising findings, the researchers caution that further studies are needed to determine if results from cultured cell experiments will translate into real-world medical applications.
Looking Ahead
This development follows a growing interest in personalized medicine, where treatments are tailored to individual genetic profiles. The ability to manipulate DNA repair pathways could enhance the precision of CRISPR therapies, making them more effective and safer for patients.
As CRISPR technology continues to evolve, the implications of this study may extend beyond cancer treatment, potentially impacting a wide range of genetic disorders. The research underscores the importance of understanding drug interactions in the context of advanced therapies, paving the way for more informed clinical decisions.
Meanwhile, the scientific community eagerly awaits further validation of these findings, which could herald a new era in precision medicine, offering hope to patients worldwide.







