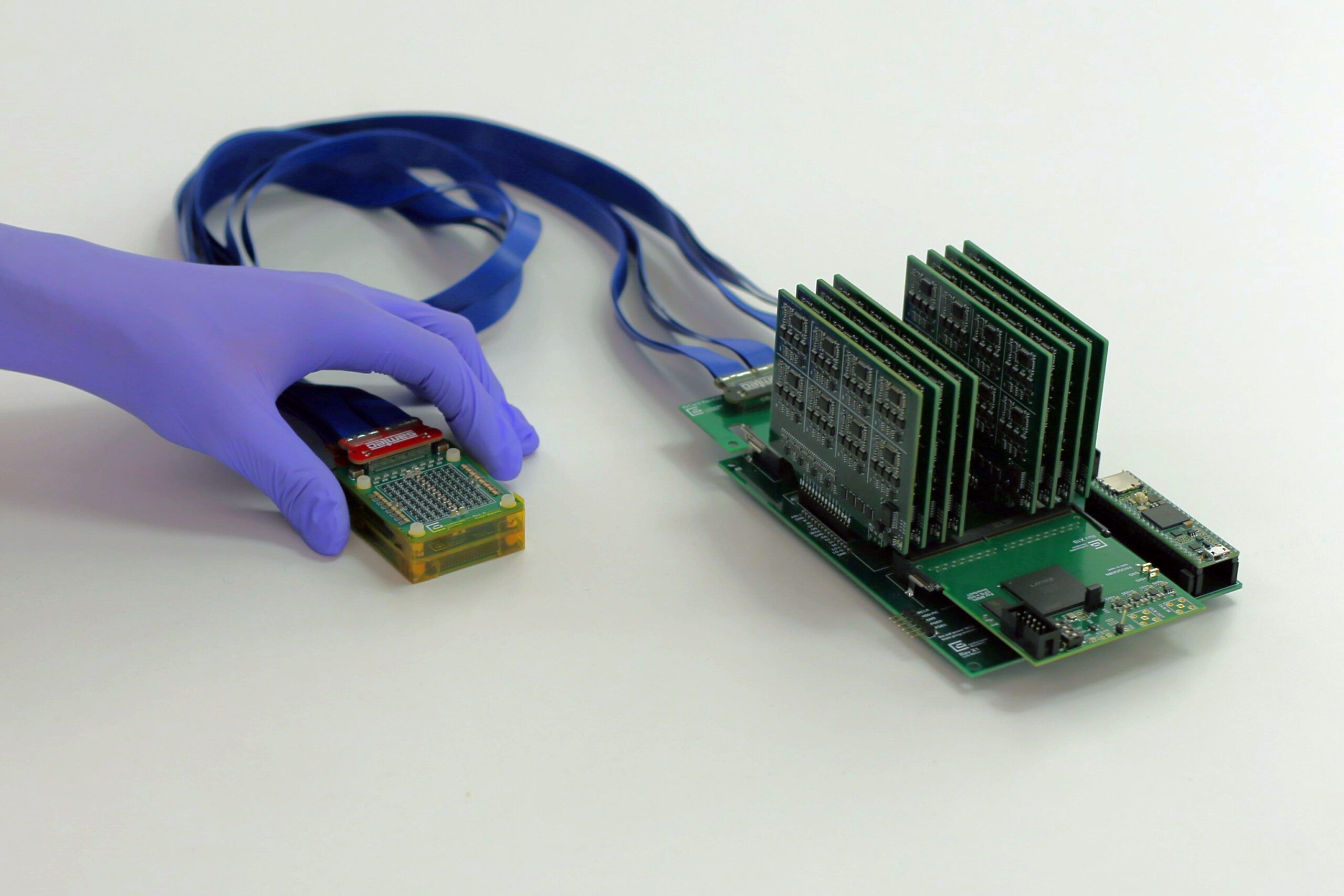
Bioengineers at the University of California San Diego have unveiled a groundbreaking technology capable of mapping the entire network of RNA-protein interactions within human cells. This pioneering advancement holds the potential to revolutionize treatment strategies for a spectrum of diseases, including cancer and Alzheimer’s.
RNA-protein interactions are pivotal in regulating essential cellular processes, from gene expression to stress response. Historically, scientists have only been able to capture limited subsets of these interactions, leaving much of the cellular “conversation” obscured. Sheng Zhong, a professor in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering, describes the new technology as akin to a “wiring map” of cellular dialogues.
“This technology is like a wiring map of the cell’s conversations,” said Sheng Zhong. “It shows which RNAs are physically talking to which proteins. Many diseases, including cancer and neurodegeneration, arise when these conversations push cells to do the wrong things, like grow when they shouldn’t, ignore stress signals, or evade the immune system. Once we can see the specific RNA-protein chats that matter, we can design medicines to quiet or reroute them.”
Unveiling the Cellular Conversations
The innovative technology operates by essentially capturing the precise moment when RNAs and proteins interact within cells. Each protein is tagged and chemically linked to its corresponding RNA, which are then converted into unique DNA barcodes. These barcodes are deciphered using standard sequencing machines, resulting in a comprehensive catalog of RNA-protein interactions from a single experiment.
When applied to two human cell lines, the technology revealed over 350,000 interactions, including numerous previously unobserved ones. Zhong’s team not only confirmed known RNA-binding proteins but also identified hundreds of unexpected interactions.
Unexpected Discoveries and Implications
Among the surprising findings was the interaction involving phosphoglycerate dehydrogenase (PHGDH), an enzyme previously identified by Zhong’s team as a causal gene in Alzheimer’s disease and a potential blood biomarker for early detection. In this study, PHGDH was found to bind messenger RNAs associated with cell survival and nerve growth, suggesting additional ways it may influence brain health.
Additionally, the research uncovered that the long noncoding RNA LINC00339 interacts with 15 membrane proteins. Given that this RNA is elevated in several cancers, these interactions may provide insights into how it drives tumor growth and metastasis.
Potential for Drug Development
The ability to visualize these hidden interactions could pave the way for new drug targets and treatment strategies. As Shuanghong Xue, a postdoctoral scholar and co-first author of the study, explains, “Interactions that act as control knobs for disease become drug targets — either the RNA, the protein partner, or the contact surface between them. If certain RNA-protein interactions promote disease, blocking them would be a potential therapeutic strategy. If other interactions protect against disease, we’d aim to preserve or enhance them.”
Importantly, the technology not only identifies RNA-protein interactions but also pinpoints the specific regions of proteins involved and the RNA sequences preferred by each protein. This level of detail provides crucial entry points for designing targeted therapies.
Future Research Directions
While the technology represents a significant scientific advance, much work remains to be done. “For most of the new interactions we’ve uncovered, their exact biological roles still need to be worked out,” said Zhong. “The main advance here is that we’ve created a comprehensive, unbiased map of potential RNA-protein partnerships. This opens the door for future research to figure out which ones drive disease, which ones are protective, and how we can target them with drugs.”
Zhong’s team is now applying the technology to disease models, including Alzheimer’s and Parkinson’s, with the aim of identifying malfunctioning RNA-protein interactions that could form the basis for next-generation therapies.
Full study: “Genome-wide mapping of RNA-protein associations through sequencing.” Co-first authors Zhijie Qi and Shuanghong Xue contributed key innovations in bioinformatics, AI tools, and molecular interactions.
This research is supported by the National Institutes of Health, under grants R01GM138852, DP1DK126138, UH3CA256960, and R01HD107206.