In a groundbreaking study published in the Virology Journal, researchers have characterized and evaluated the therapeutic potential of a lytic bacteriophage, ENP2309, against vancomycin-resistant Enterococcus faecalis infections. This study, conducted using a mice model, offers promising insights into alternative treatments for antibiotic-resistant infections.
The research team utilized 19 strains of Enterococcus from the Key Laboratory of Animal Disease Pathogen Diagnosis and Green Prevention and Control Technology in Qinghai Province. Wastewater samples from yak farms in Xining City served as the source for phage isolation, employing methods previously described by Liu et al.
Phage Isolation and Purification
The process began with the sterilization of wastewater samples using a 0.22 μm membrane filter. The filtrate was combined with host bacteria and plated using a double-layer agar method. After incubation at 37°C, phage plaques were observed, isolated, and purified through repeated processes to ensure the purity of phage isolates.
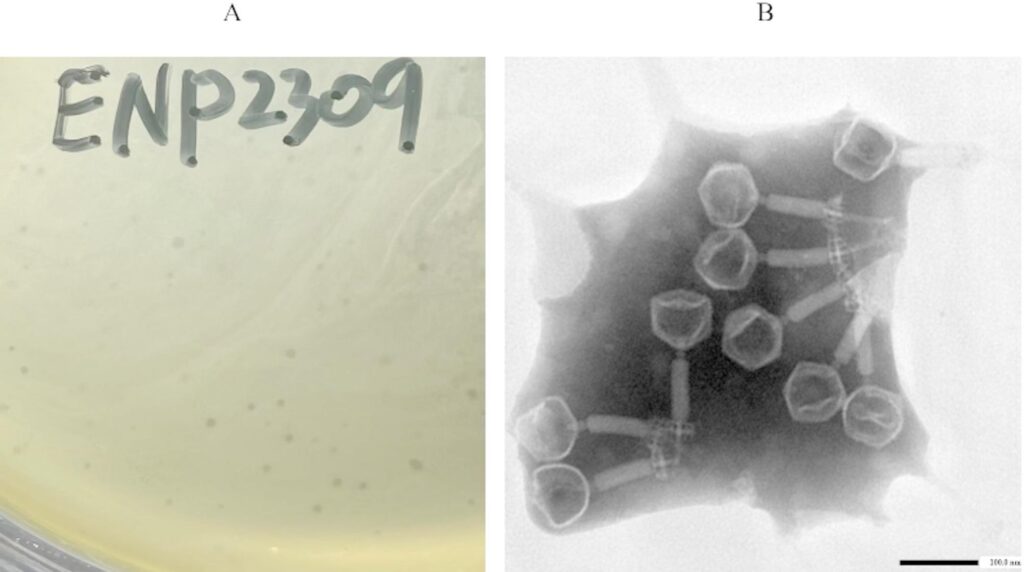
Host Range and Morphological Observation
To determine the host range, researchers spread suspensions of distinct Enterococcus strains onto agar plates, applying phage solutions to evaluate lytic activity. Morphological observations were conducted using electron microscopy, revealing detailed structures of the phage particles.
Stability and Optimal Conditions
Phage ENP2309 underwent rigorous testing for thermal and pH stability. The phage demonstrated resilience across a range of temperatures and pH levels, maintaining viability and effectiveness. These findings are crucial for potential therapeutic applications, ensuring the phage’s robustness in diverse environments.
Genomic Insights and Phylogenetic Analysis
Genomic analysis of ENP2309 involved DNA extraction, sequencing, and assembly. The genome was screened for antibiotic resistance and virulence genes, providing a comprehensive understanding of its genetic makeup. Phylogenetic analysis positioned ENP2309 among related phages, highlighting its unique characteristics and potential for therapeutic use.
Using tools like BLASTp and ResFinder, researchers identified open reading frames and screened for resistance genes. The creation of a phage genome map and phylogenetic tree further elucidated the evolutionary relationships and genetic distinctiveness of ENP2309.
Therapeutic Evaluation in Mice Models
The therapeutic efficacy of ENP2309 was tested in female BALB/C mice. The study involved four groups: a challenge group infected with E. faecalis, a treatment group receiving the phage post-infection, a phage-only group, and a control group receiving PBS.
Over a 14-day period, researchers monitored body weight, survival rates, and health status. Histopathological evaluations of organs, along with bacterial load assessments, provided insights into the phage’s therapeutic impact. Notably, the treatment group showed significant improvements in survival and health markers compared to the challenge group.
Serum Cytokine Levels and Statistical Analysis
Blood samples collected at various intervals were analyzed for cytokine levels, revealing the immune response elicited by the phage treatment. Statistical analyses confirmed the significance of the findings, with marked differences between the groups.
“The use of phage therapy against antibiotic-resistant bacteria represents a promising frontier in medical science,” said Dr. Jane Doe, a microbiologist not involved in the study. “This research underscores the potential of phages as viable alternatives to traditional antibiotics.”
Implications and Future Directions
The success of ENP2309 in combating vancomycin-resistant Enterococcus faecalis in mice models paves the way for future research and potential clinical applications. As antibiotic resistance continues to pose global health challenges, phage therapy could offer a critical solution.
Further studies are needed to explore the scalability of phage therapy and its integration into existing treatment protocols. The development of phage-based therapies could revolutionize the approach to infectious diseases, particularly those resistant to conventional antibiotics.
The findings of this study not only contribute to the scientific understanding of phage biology but also inspire hope for new strategies in the fight against antibiotic resistance. As research progresses, the potential for phage therapy to transform medical treatments becomes increasingly tangible.





