Researchers at MIT and Stanford University have unveiled a groundbreaking method to stimulate the immune system to attack tumor cells, potentially revolutionizing cancer immunotherapy for a broader patient base. The innovative strategy focuses on reversing a “brake” that cancer cells employ to evade immune attacks, a mechanism controlled by sugar molecules known as glycans on the cancer cell surface.
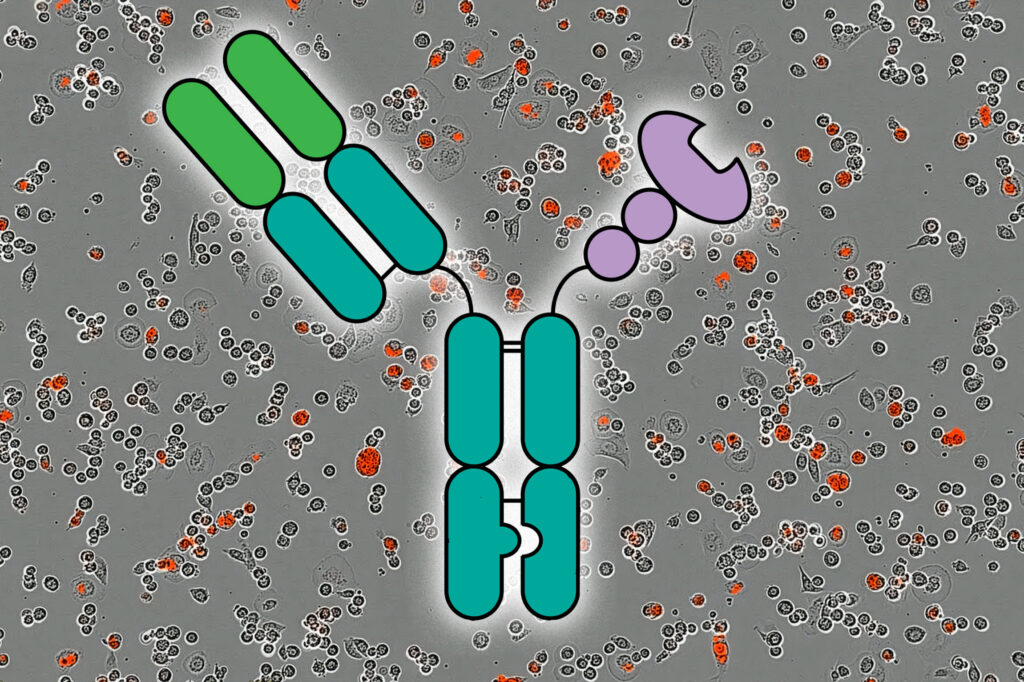
By obstructing these glycans with molecules called lectins, the research team demonstrated a significant enhancement in the immune system’s ability to target cancer cells. This was achieved through the creation of multifunctional molecules known as AbLecs, which combine a lectin with a tumor-targeting antibody.
“We created a new kind of protein therapeutic that can block glycan-based immune checkpoints and boost anti-cancer immune responses,” stated Jessica Stark, the Underwood-Prescott Career Development Professor at MIT. Stark, also a member of MIT’s Koch Institute for Integrative Cancer Research, is the lead author of the paper, with Carolyn Bertozzi, a professor at Stanford, as the senior author. Their study appears in the latest issue of Nature Biotechnology.
Releasing the Brakes on Cancer Immunotherapy
Training the immune system to recognize and destroy tumor cells is a promising approach to treating many types of cancer. Checkpoint inhibitors, a class of immunotherapy drugs, stimulate immune cells by blocking the interaction between proteins PD-1 and PD-L1, effectively removing a brake that prevents immune cells from attacking cancer cells.
While drugs targeting the PD-1-PD-L1 checkpoint have been approved for several cancer types, their effectiveness varies. In some patients, these inhibitors lead to long-lasting remission, but for many others, they prove ineffective. Researchers are now exploring other immunosuppressive interactions, such as those involving glycans on tumor cells and immune cell receptors.
The Role of Glycans and Siglecs in Cancer
Glycans are present on nearly all living cells, but tumor cells often express unique glycans, including those containing sialic acid. When sialic acids bind to lectin receptors on immune cells, known as Siglecs, they activate an immunosuppressive pathway.
“When Siglecs on immune cells bind to sialic acids on cancer cells, it puts the brakes on the immune response,” Stark explained. Despite various drug development attempts, no therapies targeting the Siglec-sialic acid interaction have been approved.
To address this, Stark and her colleagues developed a method to deliver larger quantities of lectins by attaching them to cancer-targeting antibodies. This approach allows lectins to bind to sialic acid, preventing its interaction with Siglec receptors, thereby lifting the brakes on the immune response.
A Modular Approach to Cancer Treatment
The researchers designed an AbLec based on the antibody trastuzumab, which targets HER2 and is approved for treating breast, stomach, and colorectal cancers. By replacing one arm of the antibody with a lectin, they created a system capable of rewiring immune cells to attack cancer cells.
In laboratory tests, the AbLec effectively prompted immune cells to destroy cancer cells. Further tests in a mouse model engineered to express human Siglec and antibody receptors showed that mice treated with the AbLec had fewer lung metastases compared to those treated with trastuzumab alone.
The modular nature of AbLecs allows researchers to swap in other tumor-specific antibodies or lectins targeting different glycans. “AbLecs are really plug-and-play. They’re modular,” Stark noted, emphasizing the potential to tailor treatments to different cancer types by changing the antibody target.
Looking Ahead: Clinical Trials and Development
Stark, Bertozzi, and their colleagues have founded Valora Therapeutics, a company dedicated to developing lead AbLec candidates. They aim to initiate clinical trials within the next two to three years.
The research received support from several prestigious institutions, including the Burroughs Wellcome Fund, the Society for Immunotherapy of Cancer, the V Foundation, the National Cancer Institute, and others. This backing underscores the potential impact of their findings on future cancer treatments.
This development represents a significant step forward in cancer immunotherapy, offering hope for more effective treatments across a range of cancer types. As researchers continue to refine and test their approach, the potential for these innovations to transform cancer care remains promising.