In a groundbreaking development, researchers have successfully measured the force exerted by a crucial cellular motor protein, KIF1A, using a novel DNA nanospring. This advancement, achieved by scientists from the University of Tokyo and the National Institute of Information and Communications Technology (NICT) in Japan, could significantly enhance the diagnosis of neurological disorders linked to KIF1A mutations.
KIF1A plays a pivotal role in the transport of materials within nerve cells. Mutations in this protein are known to cause neurological disorders, manifesting as difficulties in walking, intellectual impairments, and nerve degradation. However, accurately measuring the weakened motor performance resulting from these mutations has been a challenge until now.
The Role of KIF1A in Neurological Disorders
Neurological conditions such as KIF1A-associated neurological disorder (KAND) are particularly debilitating. Early diagnosis is critical, as it allows for timely intervention and management of symptoms. According to Professor Kumiko Hayashi from the Institute for Solid State Physics at the University of Tokyo, “KAND results from mutations in the motor protein KIF1A, and it’s been reported that some KIF1A mutants generate a motor force of less than 1 piconewton, compared to a healthy version’s 3.8 piconewtons.”
“Even a strong copy of KIF1A at 3.8 piconewtons only exerts a trillionth of the force needed to lift an apple,” said Professor Hayashi.
Previous attempts to measure these forces using optical tweezers were hindered by unclear signals and detachment of test samples. This led researchers to explore alternative methods, culminating in the use of a DNA nanospring.
Innovative Use of DNA Nanospring
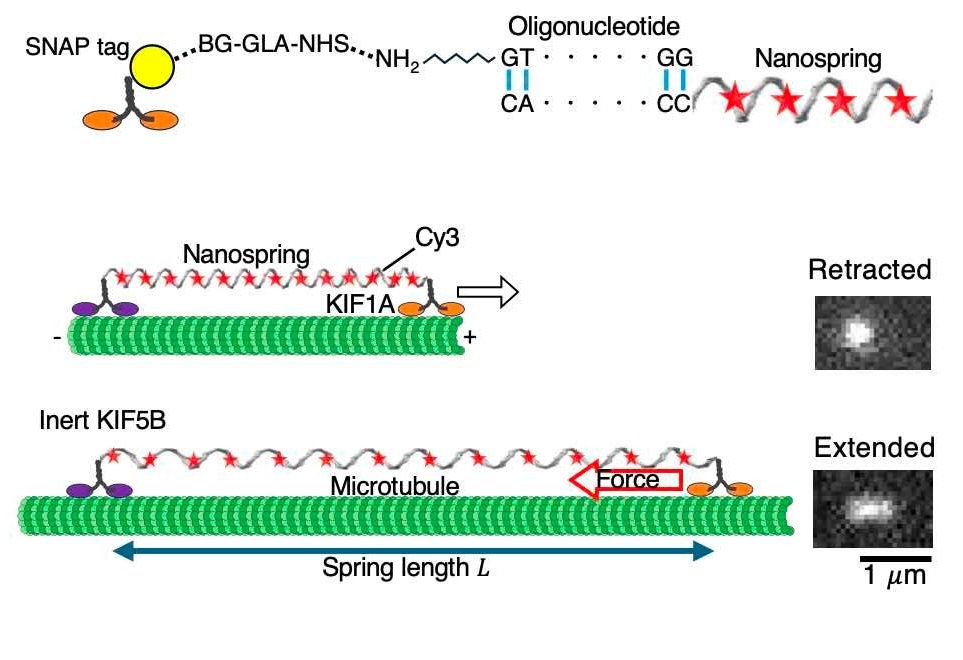
The DNA nanospring, a tiny coil only a few nanometers long, represents a significant technological innovation. Developed by Senior Researcher Mitsuhiro Iwaki from NICT, it is securely attached to both an immovable surface and a KIF1A protein. Its springlike nature allows it to extend based on the force applied, and it fluoresces under a microscope to indicate the degree of stretching.
Professor Hayashi explained, “After obtaining fluorescence images of the nanospring, it was necessary to estimate its length from the images, and we developed an estimation method to do so. Information science also proved to be important for single-molecule analysis.”
The nanosprings are made using a process called DNA origami, where a long strand of DNA is folded using many shorter strands.
This technique, aided by computer programs, allows researchers to design precise nanoscale structures. The DNA folds correctly on its own, thanks to predictable molecular interactions, enabling the creation of tiny, springlike structures with remarkable accuracy.
Implications for Diagnosis and Future Research
While the DNA nanospring itself may not directly lead to treatments, its ability to aid in diagnosing KAND marks a significant step forward. Hayashi and her team are now focused on developing high-throughput data analysis methods to catalog force measurements for over 100 known KIF1A mutations.
“Since the biophysical properties of the motor protein are important for predicting disease severity, we aim to improve predictions of KAND severity by incorporating these data into AI-based models of protein performance,” Hayashi stated.
This development follows a broader trend in biomedical research, where advances in nanotechnology and data analysis are increasingly being leveraged to tackle complex medical challenges. As researchers continue to refine their techniques, the potential for improved diagnostic tools and personalized treatment strategies grows.
Looking ahead, the integration of these findings into AI models could revolutionize how neurological disorders are understood and managed, offering hope for more effective interventions and better outcomes for patients.