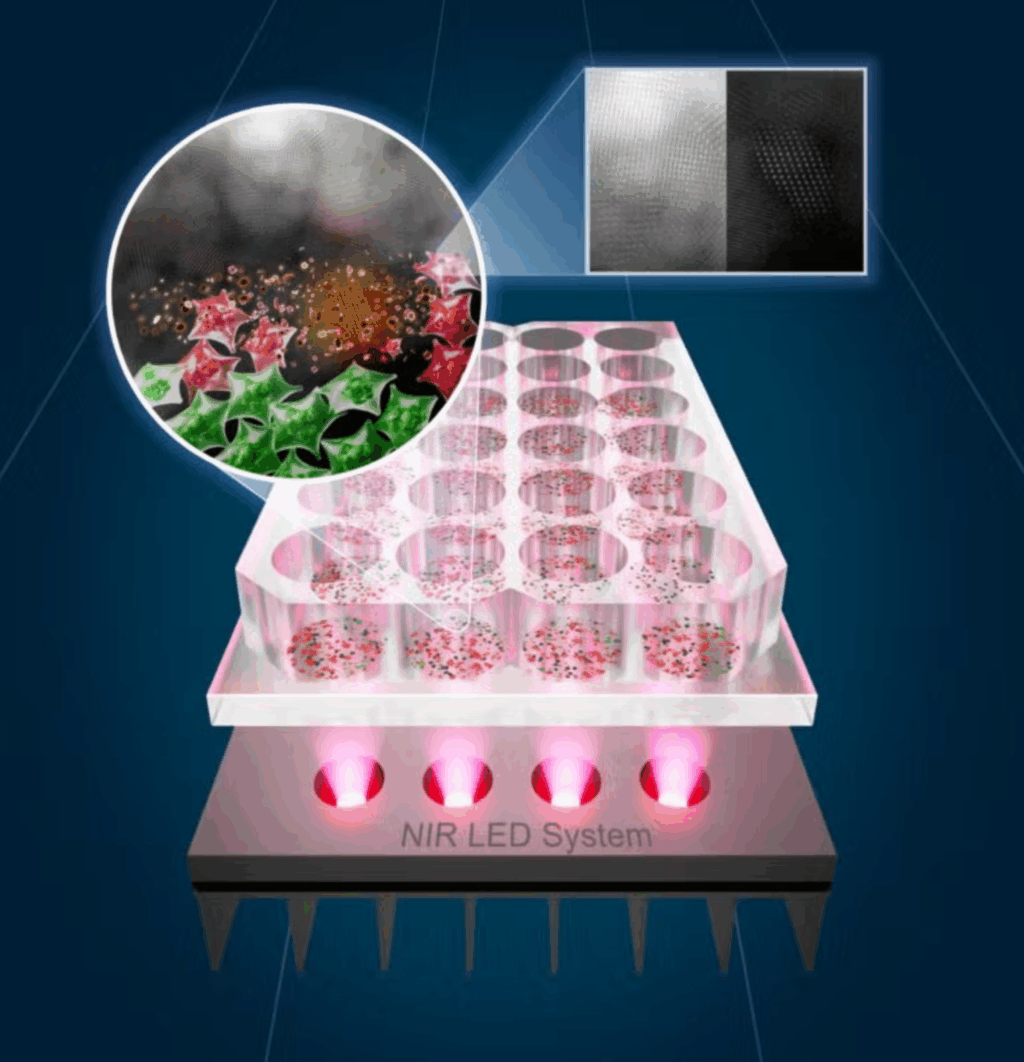
A groundbreaking study conducted by researchers at The University of Texas at Austin and the University of Porto has unveiled a novel approach to cancer treatment using tin-based nanomaterials combined with near-infrared light. This innovative method promises to deliver less toxic and more affordable cancer therapies, relying on light rather than traditional drugs or radiation.
The core of this revolutionary treatment lies in tin oxide nanoflakes, or SnOx, which are created through a straightforward electrochemical process that transforms tin disulfide (SnS₂) into a new structure. These nanoflakes absorb near-infrared (NIR) light and convert it into heat, effectively targeting and destroying cancer cells while sparing healthy ones. This photothermal therapy precisely attacks tumors by generating heat where light is concentrated.
Converting Tin into a Cancer Killer
The researchers initiated their work with layered SnS₂ nanosheets, which they electrochemically oxidized to form SnOx nanoflakes. This process introduced tiny imperfections known as oxygen vacancies, which are crucial for the material’s ability to absorb more light and emit more heat.
Electron microscopy revealed that the oxidized flakes were thinner and more porous than their predecessors, with a larger surface area for light interaction. This unique structure, combined with the novel chemical composition, made them highly effective at converting light into heat.
They rapidly heated water solutions to above 60°C, a temperature lethal to cancer cells, when irradiated with NIR at 808 nanometers, achieving a photothermal conversion efficiency of 45.6%.
Safe, Targeted, Reusable
In laboratory tests, cancer cells exposed to these nanoflakes were not highly toxic in the absence of light. However, when exposed to NIR light, the nanoflakes proved fatal for cancer cells. In experiments on colorectal and skin cancer cells, a 30-minute treatment eradicated up to 92% of skin cancer cells and half of the colorectal cancer cells, while healthy human skin cells remained unaffected.
Fluorescence microscopy showed the nanoflakes penetrating cancer cells and diffusing through their cytoplasm. Once excited, the flakes emitted bursts of heat that broke through cell membranes, causing apoptosis—cell death—without harming surrounding tissue.
The materials demonstrated resilience against prolonged use. Even after multiple heating and cooling cycles, their structure and function remained stable. In water storage, they did not clump and retained their optical properties, ensuring their stability for medical applications.
LEDs Instead of Lasers
One of the most significant advancements in this study was the replacement of expensive, high-power lasers with light-emitting diodes (LEDs). LEDs offer a more affordable and safer energy source, making the treatment easier to scale and safer for clinical use.
“It’s our aspiration to bring this technology to patients worldwide,” stated Artur Pinto, a scientist at the University of Porto and co-senior author of the study.
Pinto envisions a handheld LED device for skin cancer that patients could use at home following surgery. This tiny patch-like device would provide site-specific light treatment to eliminate any remaining cancer cells and reduce the risk of recurrence. This shift towards LED-based therapy could bring light-based treatments into hospitals and homes, particularly in regions lacking large medical equipment.
The Science Behind the Glow
The success of the nanoflakes lies in oxygen vacancies—tiny spaces in the atomic lattice that alter the material’s reaction to light. These vacancies create new electronic states, enhancing the range of NIR wavelengths absorbed, leading to improved photon capture and heat generation.
Computer modeling confirmed that oxidized tin oxide has a lower bandgap, allowing it to absorb more light energy and release it as heat rather than reflected light. This synergy of nanostructure design and defect engineering makes the material both efficient and versatile. Researchers can even customize its response by adjusting oxidation conditions.
Compared to other photothermal materials, such as gold nanorods or carbon materials, SnOx nanoflakes are cost-effective, chemically stable, and eco-friendly. The entire fabrication process occurs at room temperature without toxic chemicals, offering a green and scalable approach to medical nanomaterial production.
Looking Ahead
The researchers plan to further investigate the nanoflakes by testing them in live animal models to assess their circulation in the body and determine long-term safety. They are also exploring ways to blend the particles with other materials used as catalysts for broader medical applications.
The technology’s potential extends beyond cancer treatment. The same light-activated heat could be used in antimicrobial therapy or wound healing, where focused heat accelerates recovery.
“This is a step towards light-based medicine that’s not only powerful but practical,” summarized Jean Anne Incorvia, a professor at UT Austin’s Cockrell School of Engineering.
Practical Implications of the Research
The SnOx nanoflakes represent a breakthrough in cancer treatment, offering a noninvasive, targeted, and cost-effective alternative therapy. The combination of LED light and tin nanoflakes will make photothermal therapy safer and more accessible to hospitals and clinics worldwide.
Portable devices could eventually bring post-surgery light therapy into patients’ homes, particularly for surface cancers such as melanoma. This research contributes to making cancer treatment more bearable for patients by reducing reliance on chemotherapy and radiation, lowering costs, and easing infrastructure demands. It offers hope to patients in both advanced and resource-limited healthcare systems.
Research findings are available online in the journal ACS Nano.







