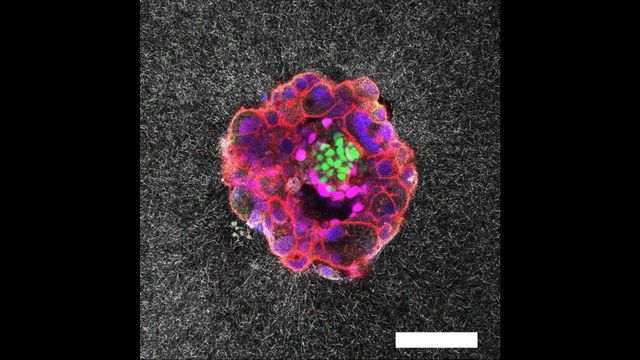
In a groundbreaking study, researchers at the Institute for Bioengineering of Catalonia (IBEC) have, for the first time, captured the intricate process of human embryo implantation in real time and in 3D. This pioneering work, published in the journal Science Advances, utilizes an innovative laboratory system that simulates the outer layers of the uterus, offering unprecedented insights into a critical phase of human reproduction.
Implantation failure is a leading cause of infertility, accounting for approximately 60% of miscarriages. By visualizing this process, the research aims to enhance understanding of the mechanisms involved, potentially improving fertility rates and optimizing assisted reproduction techniques.
Revolutionizing Reproductive Science
The collaboration between IBEC and the Reproductive Medicine Department at Dexeus Mujer–Hospital Universitari Dexeus has yielded unparalleled images of a human embryo embedding itself into the uterine tissue. This marks the first instance of such an event being recorded in real time.
Until now, the implantation process had only been observed through static images captured at specific moments. The new findings reveal that embryos exert significant force to integrate with the uterine tissue, a process previously unobserved in this detail.
“We have observed that human embryos burrow into the uterus, exerting considerable force during the process. These forces are necessary because the embryos must be able to invade the uterine tissue, becoming completely integrated with it. It is a surprisingly invasive process,” explained Samuel Ojosnegros, principal investigator of the IBEC’s Bioengineering for Reproductive Health group.
Understanding the Mechanics of Implantation
To advance during implantation, the embryo releases enzymes that break down surrounding tissue. This process requires force to penetrate the fibrous layers of the uterus, which are rich in collagen. The embryo then begins forming specialized tissues that connect to the mother’s blood vessels, establishing a vital nutrient supply.
The study’s findings indicate that embryos exert traction forces on their environment, effectively remodeling it. According to Amélie Godeau, a researcher in the Ojosnegros group, “We observe that the embryo pulls on the uterine matrix, moving and reorganizing it. It also reacts to external force cues. We hypothesize that contractions occurring in vivo may influence embryo implantation.”
This discovery underscores the importance of mechanical forces in successful embryo implantation and suggests that optimizing these forces could enhance fertility outcomes.
Innovative Laboratory Platform
The IBEC research team developed a specialized platform to facilitate this study, allowing embryos to implant outside the uterus under controlled conditions. This platform supports real-time fluorescence imaging and analysis of the embryo’s mechanical interactions with its environment.
Constructed from a gel mimicking the uterine matrix, the platform is enriched with collagen and essential proteins for embryo development. Experiments conducted with both human and mouse embryos revealed species-specific implantation patterns, providing valuable comparative insights.
“Our platform has enabled us to quantify the dynamics of embryo implantation and determine the mechanical footprint of the forces used in this complex process in real time,” said Anna Seriola, IBEC researcher and co-first author of the study.
Implications for Future Fertility Treatments
The implications of these findings are profound, offering potential advancements in fertility treatments by improving embryo quality and reducing the time required for conception through assisted reproduction. The study also highlights the importance of selecting ideal conditions for embryo research, as emphasized by Miquel Solé, director of the Laboratory of Cryopreservation at Dexeus Mujer.
This research represents a significant leap forward in reproductive science, providing a new lens through which to view and understand the complexities of human reproduction. As scientists continue to explore these findings, the potential for improved fertility treatments becomes increasingly tangible.
For further information, please refer to the original study: Godeau AL, Seriola A, Tchaicheeyan O, et al. Traction force and mechanosensitivity mediate species-specific implantation patterns in human and mouse embryos. Sci Adv. 2025;11(33):eadr5199. doi: 10.1126/sciadv.adr5199





