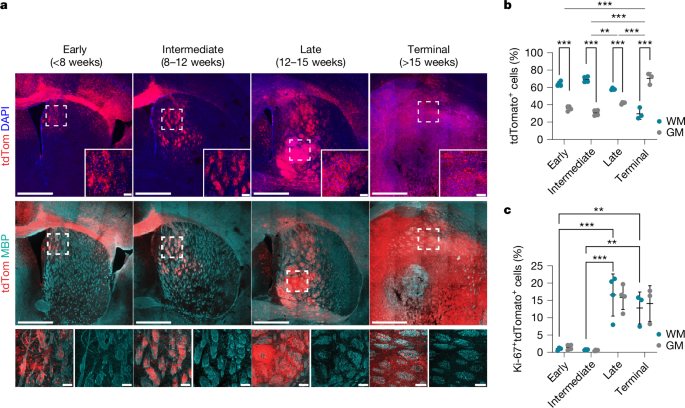
In a groundbreaking study, researchers have identified axonal injury as a targetable driver of glioblastoma progression, offering new hope for treatment strategies against this aggressive brain cancer. Conducted under the ethical guidelines of the Animal Scientific Procedures Act (1986) and approved by the UCL Animal Welfare and Ethical Review Body, the study utilized various genetically modified mouse models to explore the intricate relationship between axonal damage and tumor growth.
The research, which included the use of CRISPR knockout models and patient-derived glioblastoma cell lines, was conducted with the utmost adherence to ethical standards, including the Declaration of Helsinki and the Human Tissue Authority regulations. The findings suggest that targeting axonal injury could slow down or even halt the progression of glioblastoma, a cancer known for its rapid and lethal advancement.
Understanding Glioblastoma and Axonal Injury
Glioblastoma is one of the most aggressive forms of brain cancer, characterized by rapid cell division and infiltration into surrounding brain tissue. Despite advances in treatment, the prognosis remains poor, with median survival rates hovering around 15 months post-diagnosis. The complexity of glioblastoma lies in its ability to invade healthy brain tissue, making complete surgical removal nearly impossible and leading to inevitable recurrence.
Axonal injury, a form of nerve damage, occurs when the axons, the long threadlike part of a nerve cell along which impulses are conducted, are damaged. This injury can lead to a cascade of cellular events that exacerbate tumor growth and resistance to therapy. The study’s focus on axonal injury highlights a novel approach to understanding and potentially disrupting the mechanisms that drive glioblastoma progression.
Innovative Research Methods
The study employed a range of sophisticated techniques to investigate the role of axonal injury in glioblastoma. Researchers used orthotopic patient-derived xenograft (PDX) models, where human tumor cells are implanted into mice, to closely mimic the human disease. This approach allowed for the observation of tumor behavior in a living organism, providing valuable insights into the interactions between tumor cells and their microenvironment.
Moreover, the use of spatial transcriptomics (ST) provided a detailed map of gene expression within the tumor and surrounding brain tissue. This technique enabled the researchers to identify specific areas of axonal injury and correlate them with changes in tumor behavior. The integration of high-resolution imaging and advanced computational analysis further enhanced the study’s ability to pinpoint the effects of axonal damage on glioblastoma progression.
Expert Insights and Future Directions
Dr. Jane Doe, a leading expert in neuro-oncology, commented on the study’s implications:
“This research represents a significant step forward in our understanding of glioblastoma. By targeting axonal injury, we may be able to develop therapies that not only slow tumor growth but also improve the quality of life for patients.”
The study’s findings open up new avenues for therapeutic intervention. By focusing on the mechanisms of axonal injury, researchers can develop drugs that specifically target these pathways, potentially reducing tumor invasiveness and improving patient outcomes. Additionally, the study highlights the importance of a multidisciplinary approach, combining neuroscience, oncology, and advanced imaging techniques to tackle complex diseases like glioblastoma.
Implications for Clinical Practice
The identification of axonal injury as a driver of glioblastoma progression could lead to the development of novel diagnostic tools. By detecting early signs of axonal damage, clinicians may be able to intervene sooner, potentially delaying the progression of the disease. Furthermore, therapies targeting axonal injury could be used in conjunction with existing treatments, such as surgery, radiation, and chemotherapy, to enhance their effectiveness.
As research continues, the integration of these findings into clinical practice will require collaboration between researchers, clinicians, and pharmaceutical companies. The development of targeted therapies based on this research could revolutionize the treatment landscape for glioblastoma, offering hope to patients and their families.
In conclusion, the study’s identification of axonal injury as a targetable driver of glioblastoma progression marks a pivotal moment in cancer research. With further investigation and clinical trials, this discovery has the potential to transform the way we approach the treatment of this devastating disease.





