Alzheimer’s disease (AD) remains the leading cause of dementia worldwide, affecting millions of individuals and presenting a wide spectrum of symptoms. Recent studies have begun to unravel the complex genetic underpinnings of this neurodegenerative disorder, with a particular focus on the role of brain somatic mutations. These mutations, which occur in non-reproductive cells, are increasingly recognized as significant contributors to the pathogenesis of AD.
Somatic mutations in neurons have been linked to the pathological hallmarks of AD, including amyloid-beta (Aβ) protein deposition, hyperphosphorylation of Tau protein, and chronic neuroinflammation. These mutations can lead to neuronal dysfunction and progressive neurodegeneration, underscoring their potential impact on disease progression.
Genetic Discoveries in Alzheimer’s Disease
Research has identified several AD-associated genes, such as APP, PSEN1, and PSEN2, which play pivotal roles in the disease’s pathogenesis. Mutations in these genes can cause familial forms of AD, characterized by early onset and autosomal dominant inheritance patterns. These genetic discoveries have provided crucial insights into the molecular mechanisms underlying AD.
While familial AD accounts for a small percentage of cases, the majority of AD cases are sporadic, with later symptom onset and no clear familial inheritance patterns. This has led researchers to explore the role of somatic mutations, which are DNA sequence alterations occurring in non-reproductive cells during an organism’s growth and aging.
Somatic Mutations and Their Impact
Recent advances in sequencing technologies have revealed that somatic mutations are prevalent in both pathological and normal cell populations. These mutations are broadly classified into categories such as single nucleotide variants (SNVs), insertions and deletions (Indels), and structural variations (SVs). In neurons, these mutations can significantly influence cellular physiology and function.
In the context of AD, somatic mutations have been linked to various neurodegenerative disorders. For instance, somatic BRAF V600E mutations have been implicated in neurodegenerative processes, while the somatic expansion of mutated CAG repeat sequences is considered a key step in Huntington’s disease pathogenesis. Despite these advances, the relationship between somatic mutations and AD remains poorly understood.
Single Nucleotide Variants (SNVs)
SNVs, which involve the substitution of a single nucleotide in the genome, occur ubiquitously across various cell types. In neurons, hundreds of SNVs may exist in each cell, with a strong correlation between SNV burden and age. These mutations can be categorized into distinct mutational signatures, facilitating the identification of potential triggers for point mutations.
Neurons in the prefrontal cortex and dentate gyrus display abundant C > T mutations, forming a mutation signature known as Signature B. This mutation may result from the deamination of methylated cytosine in non-replicating neurons or cell division processes. Distinguishing true mutations from amplification artifacts in single-cell sequencing is crucial for accurate detection.
Insertions and Deletions (Indels)
Indels, which affect fewer than 50 base pairs, accumulate linearly with age in both oligodendrocytes and neurons. Neurons can accumulate approximately three Indels per year, with deletions being more frequent than insertions. Elderly individuals typically harbor hundreds of Indels in their neurons, with some pathological neurons displaying thousands.
Neuronal Indels are classified into categories such as ID-A, which is strongly associated with neurodegenerative diseases, and ID-B, which appears unrelated to disease. In oligodendrocytes, the most prevalent signature is ID9, commonly observed in gliomas and other brain tumors.
Environmental and Lifestyle Factors
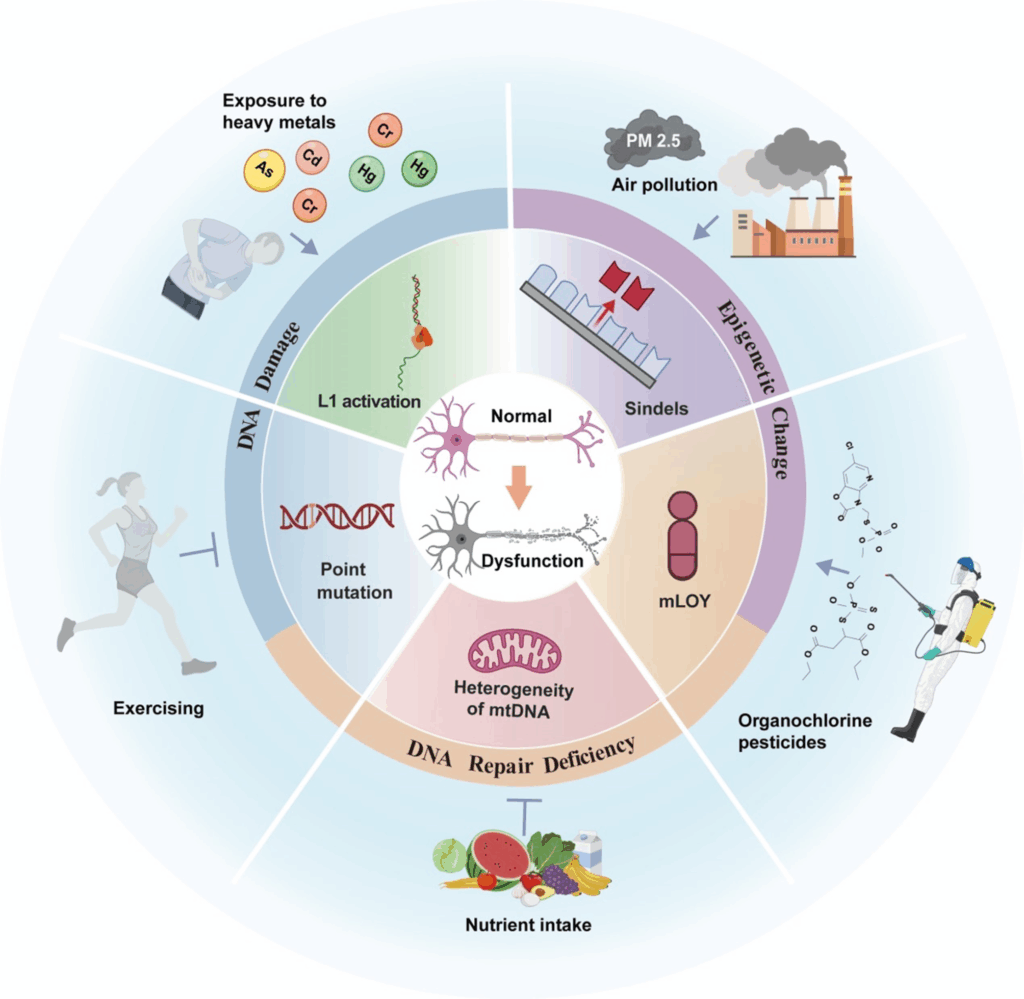
Environmental and lifestyle factors can influence the accumulation of somatic mutations. Exposure to heavy metals, air pollution, and pesticides has been linked to increased AD risk. Conversely, regular physical activity and sufficient intake of nutrients like vitamin B12 and folate can reduce AD risk and slow cognitive decline.
These factors may promote somatic mutations by damaging DNA, impairing DNA repair mechanisms, and altering the epigenetic landscape. For example, nickel chloride reduces DNA repair activity, while metals like chromium and cadmium induce oxidative stress and DNA repair defects. Understanding these processes can help elucidate the link between environmental exposures and AD.
Molecular Mechanisms
DNA damage, repair, and epigenetic alterations collectively shape somatic mutation patterns. Neurons, which lack proliferative capacity, rely heavily on DNA repair to ensure genomic accuracy. When damage exceeds repair capacity, genetic mutations and apoptosis can occur, contributing to neurodegenerative disorders.
Epigenetics plays a key role in regulating somatic mutations, with transposons being tightly controlled by epigenetic mechanisms. In AD, factors like pathogenic Tau and histone demethylase KDM4B can influence transposition through epigenetic regulation, highlighting the complex interplay between DNA damage, repair, and epigenetics.
Looking Forward
The growing body of research on somatic mutations in Alzheimer’s disease underscores the need for further investigation into their role in disease progression. By understanding the mechanisms driving these mutations and their impact on neural cells, researchers can develop new therapeutic interventions and diagnostic tools to combat AD.
As sequencing technologies continue to advance, the ability to detect and analyze somatic mutations with greater accuracy will provide deeper insights into the molecular pathology of neurodegenerative diseases. This knowledge will be crucial for developing strategies to mitigate disease progression and improve patient outcomes.