Mass General Brigham researchers have unveiled a groundbreaking artificial intelligence model named BrainIAC, capable of analyzing brain MRI datasets to perform a variety of medical tasks. These tasks include identifying brain age, predicting dementia risk, detecting brain tumor mutations, and forecasting brain cancer survival. The results of this innovative model, which outperforms existing task-specific AI models, were published in the prestigious journal Nature Neuroscience.
“BrainIAC has the potential to accelerate biomarker discovery, enhance diagnostic tools, and speed the adoption of AI in clinical practice,” stated Dr. Benjamin Kann, the corresponding author and a key figure in the Artificial Intelligence in Medicine (AIM) Program at Mass General Brigham. “Integrating BrainIAC into imaging protocols could help clinicians better personalize and improve patient care.”
Addressing Gaps in Medical AI
Despite significant advances in medical AI, there remains a scarcity of publicly available models that can broadly analyze brain MRIs. Traditional frameworks often focus on specific tasks and require extensive training with large, annotated datasets, which are challenging to obtain. Furthermore, brain MRI images vary significantly across institutions and applications, complicating the learning process for AI frameworks.
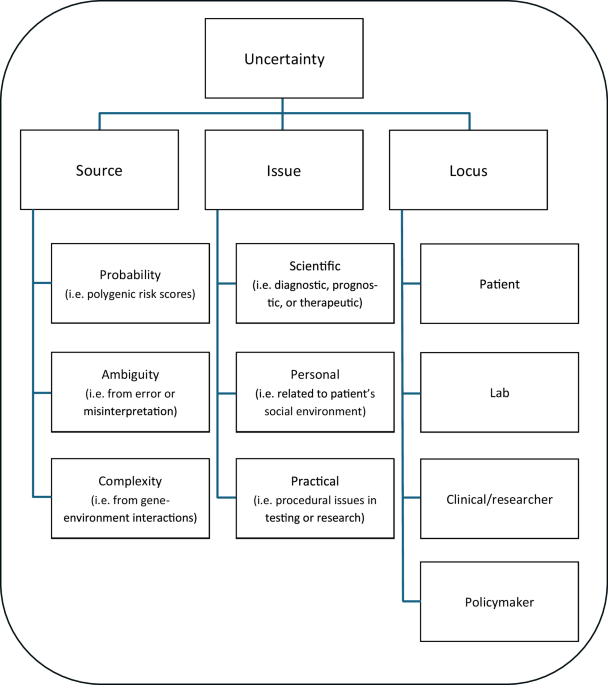
To overcome these challenges, the research team developed BrainIAC, a brain imaging adaptive core. This tool employs self-supervised learning to extract inherent features from unlabeled datasets, which can then be adapted to a wide array of applications. After pretraining on multiple brain MRI datasets, BrainIAC was validated on 48,965 diverse brain MRI scans, spanning seven distinct tasks of varying clinical complexity.
Performance and Potential
BrainIAC demonstrated its ability to generalize learnings across both healthy and abnormal images, successfully applying them to tasks ranging from classifying MRI scan types to detecting brain tumor mutation types. It outperformed three conventional, task-specific AI frameworks in these applications and others.
“BrainIAC was especially effective at predicting outcomes when training data was scarce or task complexity was high,” the researchers noted, highlighting the model’s adaptability to real-world settings where annotated medical datasets are not always available.
Further research is necessary to test BrainIAC on additional brain imaging methods and larger datasets, but its current performance suggests a promising future in medical diagnostics.
Contributors and Support
The study was authored by a team from Mass General Brigham, including Dr. Kann, Divyanshu Tak, Biniam A. Garomsa, and others. Additional contributors include Sri Vajapeyam, Maryam Mahootiha, and several more experts in the field. The research received support from the National Institute of Health/National Cancer Institute and the Botha-Chan Low Grade Glioma Consortium, among others.
Access to imaging and clinical data was provided by the Children’s Brain Tumor Network, and additional funding came from the ASCO Conquer Cancer Foundation and the Radiation Oncology Institute.
Implications for the Future
The introduction of BrainIAC marks a significant advancement in the use of AI for medical imaging. By enabling more comprehensive and flexible analysis of brain MRIs, this model could transform diagnostic processes and patient care. As AI continues to evolve, models like BrainIAC may pave the way for more personalized and efficient healthcare solutions.
Looking ahead, the research team plans to expand the application of BrainIAC to other imaging modalities and explore its integration into clinical workflows. The potential for AI to revolutionize the medical field is vast, and BrainIAC stands at the forefront of this transformative journey.