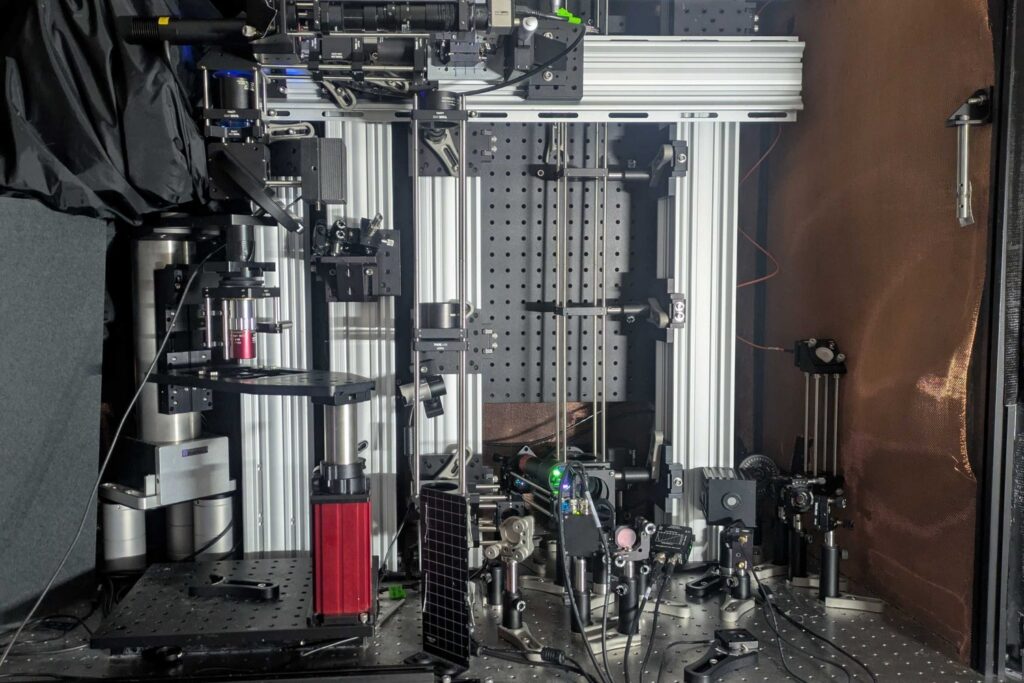
In a groundbreaking development for neuroscience and medical research, a team of MIT scientists and engineers has unveiled a revolutionary microscope system capable of imaging brain tissues at the molecular level. This new technology allows researchers to observe individual cell activity deep within the brain, including regions like the hippocampus, by utilizing sound waves.
The announcement comes as scientists continue to push the boundaries of microscopy, striving for deeper and sharper images of brain activity. The study, published in the journal Light: Science and Applications, demonstrates the system’s ability to detect NAD(P)H, a molecule closely linked to cell metabolism and neuronal electrical activity, in samples such as a 1.1-millimeter “cerebral organoid” and a 0.7-millimeter-thick slice of mouse brain tissue.
Innovative Imaging Techniques
“The major advance here is to enable us to image deeper at single-cell resolution,” explained Mriganka Sur, a neuroscientist and corresponding author of the study. Sur collaborated with mechanical engineering professor Peter So and principal research scientist Brian Anthony to develop this cutting-edge technology.
Co-lead author and mechanical engineering postdoc W. David Lee, who conceived the microscope’s design, remarked on the system’s potential to image even deeper, limited only by the size of the test samples. “That’s when we hit the glass on the other side,” he noted. “I think we’re pretty confident about going deeper.”
“A depth of 1.1 millimeters is more than five times deeper than other microscope technologies can resolve NAD(P)H within dense brain tissue.”
The system achieves this remarkable depth and clarity by combining advanced technologies to excite the molecule and detect the resulting energy without the need for external labels. The process involves using “three-photon” excitation, which penetrates deeper into tissue with less scattering due to the longer wavelength of the light.
Technical Insights and Applications
Unlike traditional methods that rely on near ultraviolet light, this new approach uses a burst of light at three times the normal absorption wavelength. This technique allows the excitation to produce a weak fluorescent signal, while most absorbed energy results in localized thermal expansion within the cell, generating sound waves. A sensitive ultrasound microphone detects these waves, which are then transformed into high-resolution images through software, akin to a sonogram.
“We merged all these techniques – three-photon, label-free, photoacoustic detection,” said co-lead author Tatsuya Osaki. “We integrated all these cutting-edge techniques into one process to establish this ‘Multiphoton-In and Acoustic-Out’ platform.”
The team also demonstrated the system’s ability to perform simultaneous “third-harmonic generation” imaging, which provides detailed cellular structures alongside photoacoustic imaging. This method could potentially detect other molecules, such as the genetically encoded calcium indicator GCaMP, used by neuroscientists to signal neural electrical activity.
Future Prospects and Clinical Implications
With the concept of label-free, multiphoton, photoacoustic microscopy (LF-MP-PAM) now established, the team is looking towards practical applications in neuroscience and clinical settings. NAD(P)H imaging has already shown promise in wound care through the company Precision Healing, Inc., founded by Lee. In the brain, variations in NAD(P)H levels are associated with conditions like Alzheimer’s disease, Rett syndrome, and seizures, making it a valuable biomarker.
The move represents a significant step forward, as the system’s label-free nature allows for potential use during brain surgeries in humans. The next challenge for the team is to demonstrate the technology in a living animal, which requires the microphone to be positioned above the sample, alongside the light source.
Lee expressed confidence in the system’s capabilities, stating that imaging at depths of 2 millimeters in live brains is feasible. “In principle, it should work,” he affirmed.
The research, supported by the National Institutes of Health, the Simon Center for the Social Brain, and other institutions, marks a pivotal moment in the field of brain imaging. As the technology continues to evolve, it holds the promise of transforming our understanding of brain function and disease.