Pennsylvania State University researchers have uncovered a novel pathway for a fundamental chemical reaction, potentially revolutionizing industrial processes and environmental management. The discovery, detailed in the Journal of the American Chemical Society, reveals that the oxidative addition reaction can follow an alternative route, challenging traditional chemical paradigms and opening new avenues for chemical design.
The study, led by Jonathan Kuo, assistant professor of chemistry at Penn State, suggests that this alternative pathway may have been occurring unnoticed, offering new insights into chemical reactions involving transition metals. These metals, such as platinum and palladium, play a crucial role in modifying the electron structures of organic compounds, thereby enabling a broader range of reactions.
Transition Metals: Breaking the Rules of Organic Chemistry
Organic compounds, primarily composed of carbon, hydrogen, and oxygen, have limited bonding patterns due to their specific electron arrangements. Transition metals, however, possess additional electron arrangements, allowing them to interact with organic compounds in complex ways. This interaction can lead to the breaking of chemical bonds and catalyzing reactions that are otherwise impossible with purely organic compounds.
“Transition metals have properties that allow them to ‘break the rules’ of organic chemistry,” Kuo explained. “Even though biological systems are largely organic, much of the chemistry in cells occurs at active sites where metallic co-factors drive reactivity. Transition metals are also vital in catalyzing industrial-scale chemical reactions.”
Understanding Electron Dynamics in Chemical Reactions
Chemical reactions occur because atoms seek more stable states, primarily through electron rearrangement among orbitals. For instance, two hydrogen atoms can bond to form dihydrogen (H2), creating hybrid orbitals that result in energy savings and increased stability. Larger elements with multiple orbitals, such as s-, p-, d-, and f-orbitals, offer greater diversity in electronic structure, leading to more varied chemical reactions.
Kuo noted, “In nature, a hydrogen atom can only support its electron using its only orbital resource, the 1s orbital. But when combined, two hydrogen atoms can efficiently share their orbital resources.”
Redefining Oxidative Addition
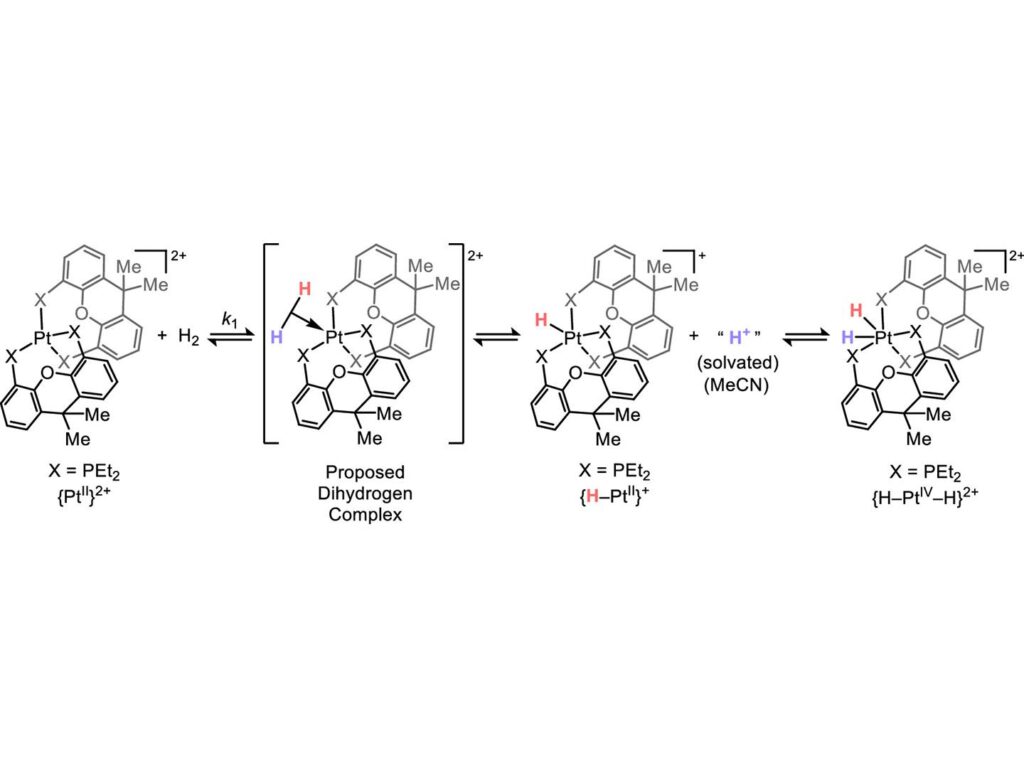
Traditionally, oxidative addition involves transition metals donating electrons to organic substrates. This electron donation facilitates various reactions. However, Kuo’s team discovered a subgroup of oxidative additions accelerated by electron-deficient transition metal compounds. In this scenario, electrons move from the organic molecule to the transition metal, a process known as heterolysis, which had not been previously observed to result in oxidative addition.
The research team used platinum and palladium compounds, exposing them to hydrogen gas and monitoring changes via nuclear magnetic resonance spectroscopy. This approach revealed an intermediate step where hydrogen donated electrons to the metal complex, culminating in a state indistinguishable from oxidative addition.
“We are excited to add this new play to the transition metal playbook,” Kuo said. “Showing that this can occur opens up new and exciting ways we might use transition metal chemistry. I am especially interested in finding reactions that could break down stubborn pollutants.”
Implications and Future Directions
This groundbreaking discovery not only challenges existing chemical theories but also holds significant implications for industrial and environmental applications. By leveraging the unique properties of transition metals, chemists can enhance the efficiency of industrial processes and develop novel solutions to reduce environmental pollutants.
As Kuo and his team continue to explore the potential of transition metal chemistry, the scientific community anticipates further breakthroughs that could transform the landscape of chemical reactions. The research, supported by the Penn State Eberly College of Science, highlights the importance of fundamental research in driving innovation and addressing global challenges.
In addition to Kuo, the research team includes first author Nisha Rao, a graduate student in chemistry at Penn State. Their collaborative efforts underscore the critical role of academic research in advancing our understanding of complex chemical processes.
As the scientific community delves deeper into this newly uncovered pathway, the potential for groundbreaking applications in various industries remains vast and promising.