Emerging scientific research suggests that an imbalanced gut may have far-reaching effects beyond digestive issues, potentially contributing to heart failure. This revelation offers hope for new therapies targeting the microbiome, a promising frontier in cardiovascular treatment.
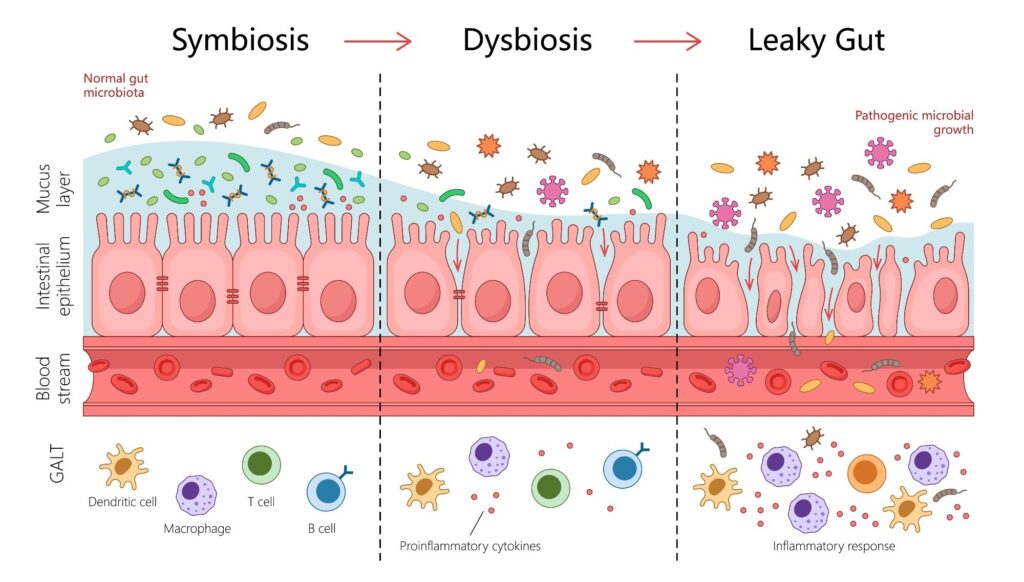
In a comprehensive review published in the journal Heart Failure Reviews, researchers analyzed nearly 50 peer-reviewed studies exploring the links between heart failure (HF) and gut dysbiosis—an imbalance in gut microbial composition. Their findings suggest that gut dysbiosis can lead to a compromised “leaky” gut barrier, allowing bacterial toxins and harmful metabolites like trimethylamine N-oxide (TMAO) to enter the bloodstream. This process drives systemic inflammation and directly damages the heart, underscoring the importance of gut microbial health in cardiovascular outcomes.
The Gut-Heart Axis: A New Understanding
The concept of a “gut-heart axis” is gaining traction among scientists, providing a new framework for understanding cardiovascular disease (CVD). This paradigm shift opens the door for novel treatments, ranging from targeted diets to microbial therapies. Heart failure, a serious condition where the heart cannot pump enough blood to meet the body’s needs, is a growing public health concern. It often co-occurs with other cardiovascular diseases, highlighting the need for improved understanding of its risk factors.
While lifestyle factors like diet, sleep, and smoking have long been associated with heart failure, the role of the gut microbiome has been less understood. Traditionally, the gut microbiome was thought to influence only digestive processes. However, a growing body of literature suggests that gut health is intricately linked to cardiovascular wellness.
Insights from Recent Research
The recent review aims to synthesize current scientific understanding of the gut-heart axis, exploring how an imbalanced microbiome can contribute to heart failure development and progression. Study data were gathered from major scientific repositories, including PubMed, ScienceDirect, and Springer, focusing on publications from the last 12 years to ensure relevance.
The findings reveal a bidirectional cascade of events starting in the gut and culminating in heart failure. Specifically, heart failure patients experience reduced blood flow to the gut, compromising the intestinal wall’s integrity and resulting in a “leaky gut.” This allows harmful microbes and metabolites to enter the bloodstream, causing systemic inflammation that exacerbates cardiovascular diseases.
Key Players in Gut-Driven Heart Failure
A critical bacterial offender identified in the research is lipopolysaccharide (LPS), a component of the outer cell membrane of gram-negative bacteria. When LPS enters the bloodstream, it binds to receptors on cardiac muscle cells, triggering a pro-inflammatory response that damages heart tissue. Microbial metabolites like TMAO, produced by gut bacteria digesting nutrients found in red meat, eggs, and fish, have been strongly associated with adverse cardiovascular outcomes.
Studies show TMAO is linked to atherosclerosis, inflammatory pathway activation, and impaired cardiac function.
Beneficial metabolites, such as short-chain fatty acids (SCFAs) like butyrate, are produced by commensal bacteria and have protective effects. However, heart failure patients often show depletions in these bacterial populations, worsening gut dysbiosis and cardiovascular health.
Potential Therapeutic Avenues
While the exact relationship between gut alterations and heart failure progression remains unclear, early research suggests potential for microbiota interventions. Trials have shown mixed results for antibiotics and probiotics in improving cardiac function, but small studies indicate some promise. Natural compounds like allicin from garlic are also being explored for their ability to modulate TMAO production.
Implications for Future Treatment
This review establishes a strong link between gut dysbiosis and heart failure, shifting the focus from a purely cardiac issue to a multi-system disorder. It highlights the gut as not just a symptom but a potential driver of disease progression. This new understanding opens up novel therapeutic avenues, focusing on gut-based interventions to improve heart outcomes.
Dietary interventions, such as the Mediterranean and DASH diets, may represent the first applications of this knowledge. These diets, rich in fiber and low in animal products, help reduce TMAO substrates and support SCFA-producing bacteria. More direct microbial interventions, including prebiotics, probiotics, and fecal microbiota transplantation (FMT), are under investigation, though FMT remains experimental.
While many approaches are still in early stages, they represent a promising future for heart failure management, where treating the gut may become a standard part of routine cardiovascular care.







