Emerging scientific research is shedding light on a surprising connection between gut health and heart failure, suggesting that an imbalanced gut may be more than just a digestive concern. It could be a significant factor in cardiovascular health, potentially paving the way for innovative treatments focused on the microbiome.
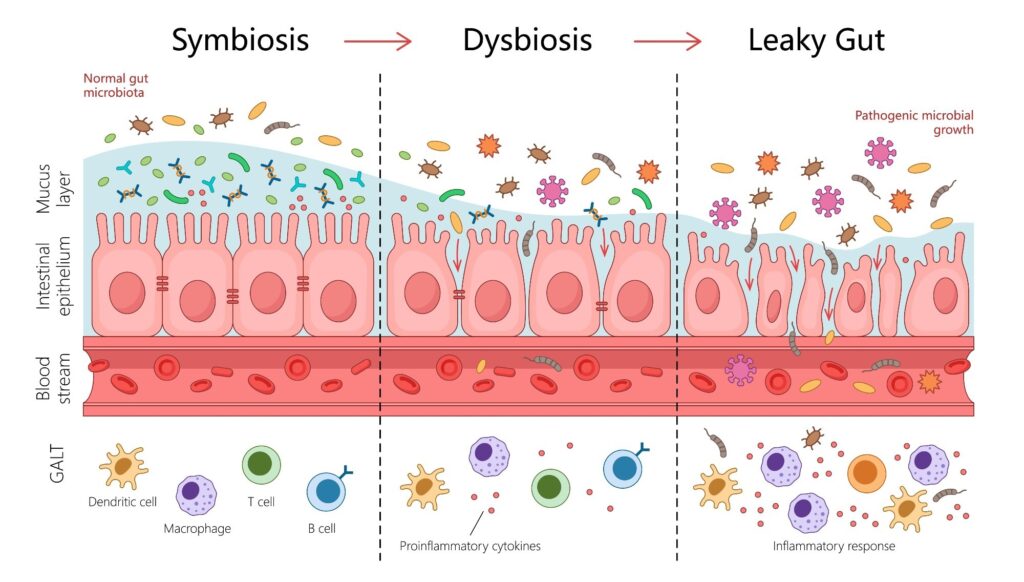
In a comprehensive review published in the journal Heart Failure Reviews, researchers analyzed nearly 50 peer-reviewed studies to explore the links between heart failure (HF) and gut dysbiosis, which is an imbalance in the gut’s microbial composition. The findings indicate that this imbalance leads to a compromised “leaky” gut barrier, allowing harmful bacterial toxins and metabolites, such as trimethylamine N-oxide (TMAO), to enter the bloodstream. This process triggers systemic inflammation and directly impacts heart health, highlighting the critical role of gut microbial health in cardiovascular outcomes.
Understanding the Gut-Heart Axis
The concept of a “gut-heart axis” is gaining traction, offering a new framework for understanding cardiovascular disease (CVD). This axis suggests that gut health is intricately linked to heart health, opening the door for novel treatments ranging from targeted diets to microbial therapies.
Heart failure is a severe cardiovascular condition where the heart cannot pump enough blood to meet the body’s needs. It is a growing public health crisis, with reports indicating its increasing global prevalence. Often co-occurring with other cardiovascular diseases such as hypertension and ischemic heart disease, heart failure necessitates a deeper understanding of its risk factors.
While traditional risk factors like diet, sleep, and smoking are well-documented, the role of the gut microbiome is less understood. However, recent literature suggests that gut health is systemically intertwined with cardiovascular wellness.
The Review’s Findings
The review synthesized current scientific understanding of the gut-heart axis, exploring how an imbalanced microbiome can contribute to heart failure’s development and progression. The study data were sourced from major scientific repositories, ensuring relevance by including only publications from the last 12 years.
One key finding is the bidirectional relationship between heart failure and gut health. Reduced blood flow to the gut in heart failure patients compromises the intestinal wall’s integrity, leading to a “leaky gut.” This condition allows microbes and harmful metabolites to enter systemic circulation, causing widespread inflammation that exacerbates cardiovascular diseases.
“A key bacterial offender identified in the research is the lipopolysaccharide (LPS) component of the outer cell membrane of gram-negative bacteria.”
When LPS enters the bloodstream, it binds to receptors on cardiac muscle cells, triggering a pro-inflammatory response. This response activates pathways that damage cytokines, contributing to cardiac fibrosis and heart function degradation.
Microbial Metabolites and Heart Health
Microbial metabolites that enter the bloodstream during a leaky gut condition have been linked to potentially life-threatening cardiovascular outcomes. TMAO, produced by certain gut bacteria when digesting nutrients like choline and carnitine, is strongly associated with adverse cardiovascular outcomes, including atherosclerosis and impaired cardiac function.
Immune cells play a crucial role in this process. Protective Treg cells, regulated by gut bacteria, are suppressed in heart failure, while pro-inflammatory Th17 cells drive cardiac inflammation. Beneficial metabolites like short-chain fatty acids (SCFAs) are produced by commensal bacteria and have protective effects, but heart failure patients often show depletions in these bacterial populations.
“A critical unanswered question remains whether these gut alterations initiate HF progression or result from established cardiac dysfunction.”
Potential Therapeutic Avenues
While trials for microbiota interventions have shown mixed results, some early-stage research offers hope. For instance, natural phytochemicals like allicin from garlic show potential for modulating TMAO production. Dietary interventions such as the Mediterranean and DASH diets, which are rich in fiber and low in animal products, may also reduce TMAO production and support beneficial bacteria.
More direct microbial interventions, including prebiotics, probiotics, and fecal microbiota transplantation (FMT), are under investigation. However, FMT remains experimental, with mixed results in animal studies.
Conclusion and Future Directions
This review establishes a strong, bidirectional link between gut dysbiosis and heart failure, shifting the focus from a purely cardiac issue to a multi-system disorder. It highlights that a compromised gut is not just a symptom but a potential driver of disease progression. This new understanding opens up novel therapeutic avenues that focus on leveraging gut-based interventions to improve heart outcomes.
While many of these approaches are still in early stages, they represent a promising and highly personalized future for heart failure management, where treating the gut may become a standard part of routine cardiovascular care.