A recent study published in PLOS Pathogens reveals that obesity significantly alters the long-term effects of COVID-19 in primates. Conducted by Charles Roberts and his team at Oregon Health & Science University, the research highlights how prior obesity and metabolic disease impact the severity and persistence of COVID-19 symptoms in rhesus macaques.
The study, which focuses on the SARS-CoV-2 delta variant, underscores the widespread nature of “long COVID” symptoms among animals, offering insights that could translate to human health. Researchers observed the effects over six months, noting that both lean and obese primates exhibited altered health parameters long after the initial infection had resolved.
Long-Term Consequences of COVID-19
Long COVID, also known as post-acute sequelae of COVID-19 (PASC), is a significant component of the COVID-19 disease burden. While it is well-established that obesity and metabolic disorders exacerbate acute COVID-19 symptoms, this study indicates that the virus also contributes to the development of new metabolic issues.
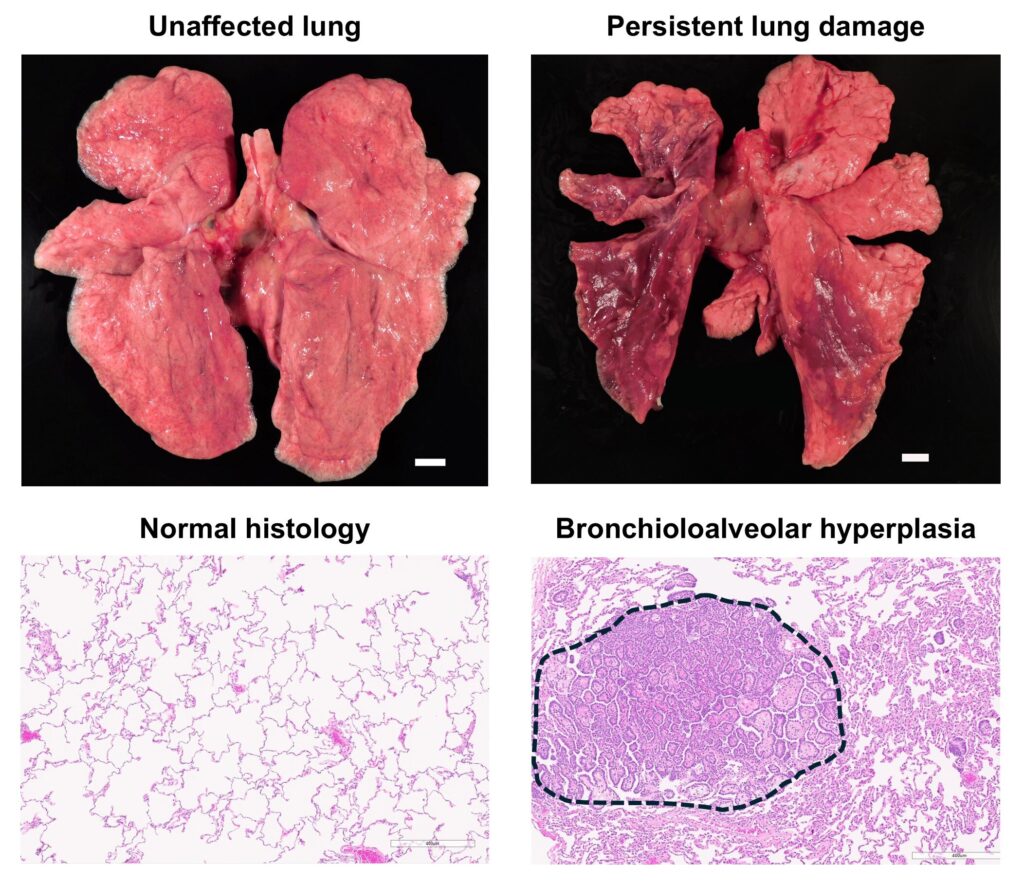
In the study, researchers compared lean and obese rhesus macaques, finding that obesity increased risks such as persistent lung damage and prolonged weight loss. Conversely, lean animals were more prone to metabolic disruptions, including a notable decline in the adiponectin to leptin hormone ratio, a key metabolic health marker.
Physiological vs. Symptomatic Effects
The research suggests that many long-term effects of COVID-19 are physiological rather than symptomatic, implying that long COVID may be more prevalent than self-reported data suggests. According to the authors, “persistent effects of SARS-CoV-2 infection are both obesity-dependent and independent.”
“Some parameters changed more robustly in obese animals, while others changed more robustly in lean animals,” the study notes.
Dr. Roberts emphasized the surprising extent of long-term adverse effects observed, even following mild initial disease courses. “This suggests that long COVID can indeed result from a mild infection,” he stated.
Implications for Future Research
The study’s findings have significant implications for ongoing and future research. Co-author Dr. Kristin Sauter highlighted the development of a macaque model for long COVID, which allows for direct comparison of different SARS-CoV-2 variants. This model is particularly valuable as it isolates the effects of the virus from pre-existing immunity complications seen in human studies.
“Our development of a macaque model of long COVID will allow us to directly compare the intrinsic differences between the effects of the delta variant described in this study with the effects of later variants such as omicron,” Dr. Sauter explained.
This research is supported by the National Institutes of Health and aims to provide a clearer understanding of how different COVID-19 variants affect long-term health outcomes.
Looking Ahead
The study’s results underscore the need for continued research into the long-term impacts of COVID-19, particularly as new variants emerge. Understanding the interplay between obesity, metabolic health, and COVID-19 could inform public health strategies and therapeutic interventions aimed at mitigating long COVID symptoms.
As the world continues to grapple with the pandemic, these findings offer a crucial perspective on the hidden, long-term consequences of the virus, emphasizing the importance of addressing obesity and metabolic health in the context of COVID-19.
For more detailed information, the study can be accessed in PLOS Pathogens, DOI: 10.1371/journal.ppat.1012988.