In a groundbreaking development, researchers from the University of California San Diego, Johns Hopkins University, UC Berkeley, and the University of São Paulo have unveiled a genetic modification in mosquitoes that blocks the transmission of malaria without affecting the insects’ health. This innovative approach, detailed in the journal Nature, could revolutionize the fight against a disease that infects millions annually and leads to nearly 600,000 deaths each year, predominantly affecting children.
Mosquitoes are the deadliest animals on the planet, with the World Health Organization reporting that in 2023 alone, they infected approximately 263 million people with malaria. Efforts to curb malaria have been hampered by the mosquitoes’ growing resistance to insecticides and the malaria parasites’ resistance to drugs. These challenges were further exacerbated by the COVID-19 pandemic, which disrupted ongoing anti-malarial initiatives.
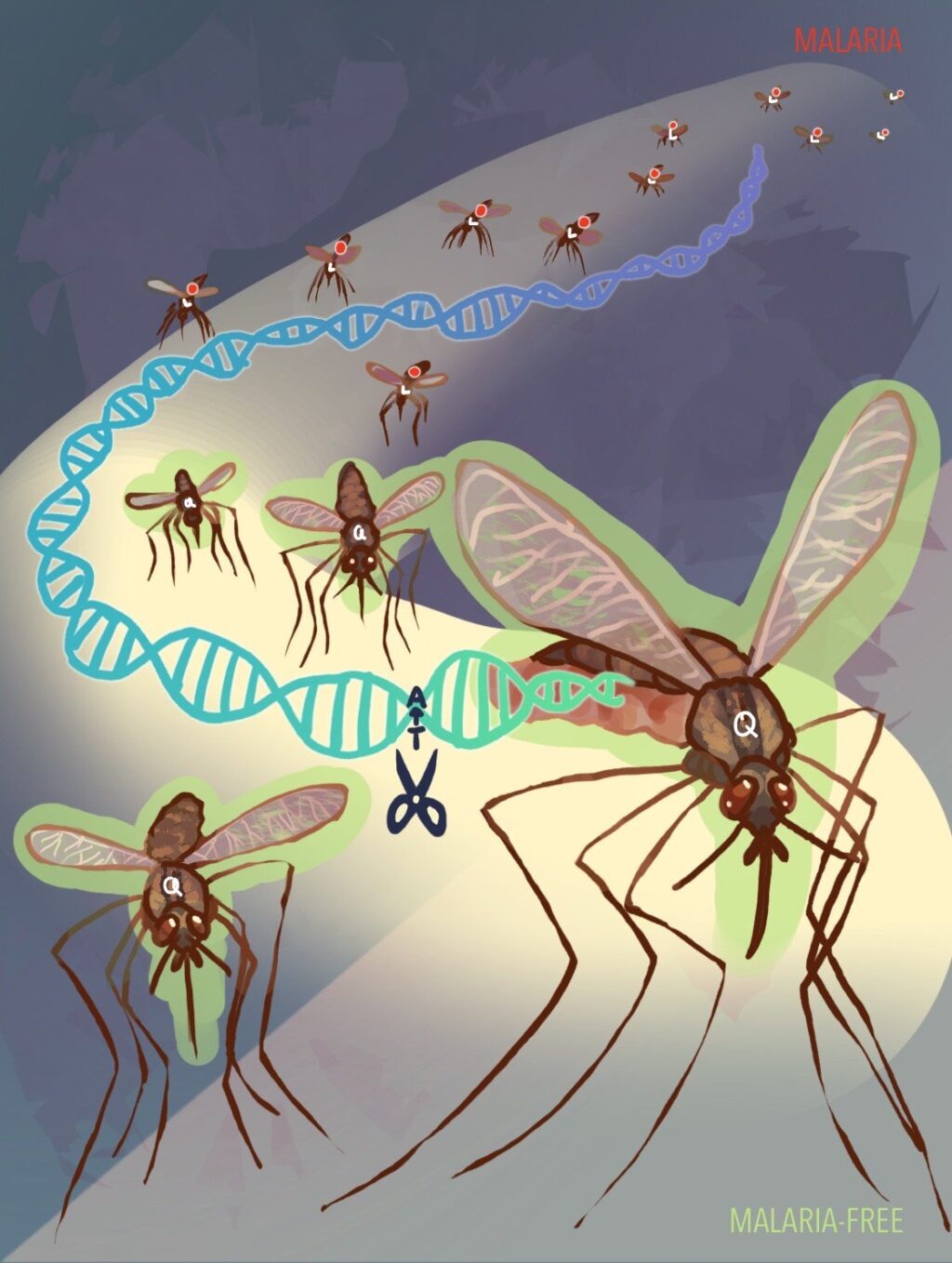
Genetic Innovation: The Allelic Gene Drive System
The new method employs a CRISPR-based gene-editing system to alter a single molecule within mosquitoes, effectively halting the malaria transmission process. The researchers, led by biologists Zhiqian Li and Ethan Bier from UC San Diego, along with Yuemei Dong and George Dimopoulos from Johns Hopkins University, have engineered mosquitoes to carry a malaria-suppressing allele, Q224, instead of the typical L224 amino acid.
“Replacing a single amino acid in mosquitoes with another naturally occurring variant that prevents them from being infected with malarial parasites—and spreading that beneficial trait throughout a mosquito population—is a game-changer,” said Bier, a professor in the UC San Diego Department of Cell and Developmental Biology.
The newly developed system uses CRISPR-Cas9 “scissors” to make a precise genetic cut, replacing the malaria-transmitting amino acid with a resistant version.
Mechanism of Action and Testing
The system targets the FREP1 protein, crucial for mosquitoes’ blood-feeding process. By switching the L224 amino acid with Q224, the researchers have effectively blocked the pathway malaria parasites use to reach the mosquitoes’ salivary glands, thereby preventing transmission to humans.
Dimopoulos, a professor at the Johns Hopkins Malaria Research Institute, tested the system on Anopheles stephensi mosquitoes, a primary vector of malaria in Asia. The results were promising, showing that the genetic tweak could block multiple malaria parasite species.
“The beauty of this approach lies in leveraging a naturally occurring mosquito gene allele,” said Dimopoulos. “With a single, precise tweak, we’ve turned it into a powerful shield that blocks multiple malaria parasite species.”
Implications and Future Prospects
Despite the genetic alteration, the mosquitoes’ growth and reproductive capabilities remained unaffected. This is a significant achievement, as the FREP1 protein plays a vital role in mosquito biology beyond its interaction with malaria parasites. The researchers have also developed a technique for the Q224 allele to be inherited by mosquito offspring, ensuring the spread of malaria resistance throughout populations.
This “allelic-drive” system is akin to a previously engineered gene-drive that reversed insecticide resistance in crop pests. Bier noted, “A similar phantom drive system could convert mosquito populations to carrying the parasite-resistant FREP1Q variant.”
Challenges and Ongoing Research
While the effectiveness of the L224-to-Q224 switch has been demonstrated, the precise mechanisms by which this change blocks malaria transmission are not yet fully understood. Ongoing research aims to uncover the underlying processes and enhance the system’s efficiency.
“This breakthrough is the result of seamless teamwork and innovation across institutions,” said Dimopoulos. “Together, we’ve harnessed nature’s own genetic tools to turn mosquitoes into allies against malaria.”
Conclusion: A New Era in Malaria Control
The development of this genetic modification marks a significant step forward in malaria control efforts. By transforming mosquitoes into vectors that cannot transmit the disease, scientists have opened new avenues for combating one of the world’s most persistent public health challenges. As research continues, the potential for widespread implementation of this technology offers hope for reducing the global burden of malaria.
For more detailed insights, the full research can be accessed in the journal Nature under the title “Driving a protective allele of the mosquito FREP1 gene to combat malaria,” authored by Ethan Bier and colleagues.







