Pennsylvania State University researchers have uncovered a novel pathway in a fundamental chemical reaction known as oxidative addition, potentially revolutionizing industrial processes and environmental solutions. The discovery, documented in the Journal of the American Chemical Society, suggests that this reaction can follow an alternative path, raising questions about its historical occurrence and opening new avenues for chemical design.
The breakthrough centers around the interactions between organic compounds and transition metals. Organic compounds, composed of elements like carbon, hydrogen, and oxygen, are traditionally limited by their specific bonding patterns and electron arrangements. Transition metals, such as platinum and palladium, offer more complex electron arrangements, which can alter the electron structure of organic compounds, leading to a broader range of possible reactions.
Understanding the Role of Transition Metals
Transition metals play a crucial role in modifying chemical reactions. According to Jonathan Kuo, assistant professor of chemistry at Penn State and leader of the research team, these metals possess unique properties that allow them to “break the rules” of organic chemistry. This capability is evident in both biological systems and industrial-scale chemical reactions.
“Transition metals have properties that allow them to ‘break the rules’ of organic chemistry,” said Jonathan Kuo. “General understanding as to how these reactions work is a way to approach the efficiency of nature or even invent reactions that don’t have a known analogy in nature.”
In nature, chemical reactions occur as atoms rearrange electrons to achieve a more stable state. This process is primarily driven by the mixing of orbitals, the regions around atomic nuclei where electrons reside. Transition metals, with their additional d-orbitals, introduce more diversity in electronic structure and potential chemical reactions.
Challenging Traditional Views of Oxidative Addition
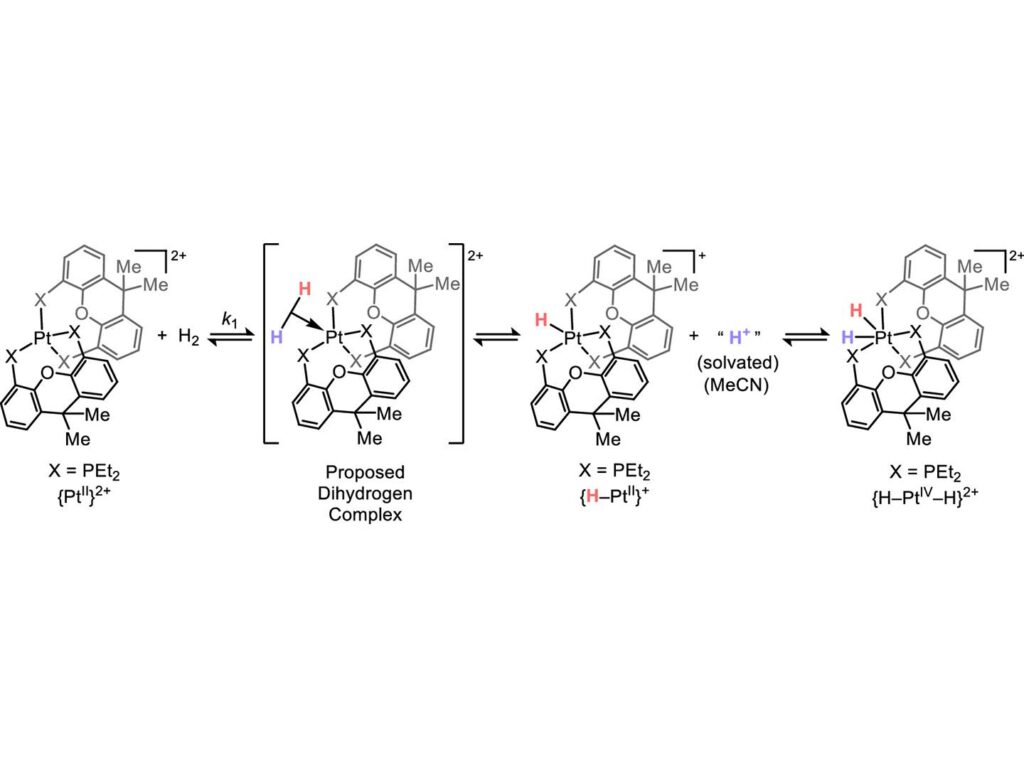
Traditionally, oxidative addition involves transition metals donating electrons to organic substrates. However, Kuo’s team has identified instances where the opposite occurs: electrons move from the organic molecule to the transition metal, a process known as heterolysis. This electron flow had not previously been observed to result in oxidative addition.
“It has, however, been noted that some oxidative additions are a little different,” Kuo explained. “A subgroup are actually accelerated by transition metal compounds that are electron deficient.”
The researchers utilized compounds containing platinum and palladium, which are not electron dense, and exposed them to hydrogen gas. Using nuclear magnetic resonance spectroscopy, they monitored changes to the transition metal complex, observing an intermediate step where hydrogen donated its electrons to the metal complex.
Implications for Industrial and Environmental Applications
The discovery of this new pathway has significant implications for industrial processes and environmental solutions. By expanding the chemical playbook, chemists can explore more efficient reactions and potentially develop methods to break down persistent pollutants.
“We are excited to add this new play to the transition metal playbook,” Kuo stated. “Showing that this can occur opens up new and exciting ways we might use transition metal chemistry. I am especially interested in finding reactions that could break down stubborn pollutants.”
Beyond its immediate applications, this research underscores the importance of understanding the diverse ways chemical reactions can occur. By leveraging the unique properties of transition metals, chemists can design more efficient industrial processes and innovative solutions to environmental challenges.
The research team, supported by the Penn State Eberly College of Science, includes first author Nisha Rao, a graduate student in chemistry at Penn State. As the scientific community continues to explore the potential of transition metals, this discovery marks a significant step forward in the field of chemistry.






