Diagnoses of attention deficit hyperactivity disorder (ADHD) are on the rise in the UK, with more children and teenagers being referred for assessment and support. This surge has put pressure on schools, health services, and parents to find effective treatments for managing symptoms like attention deficits, impulsivity, and hyperactivity. However, families often face long waits and limited options once a diagnosis is made.
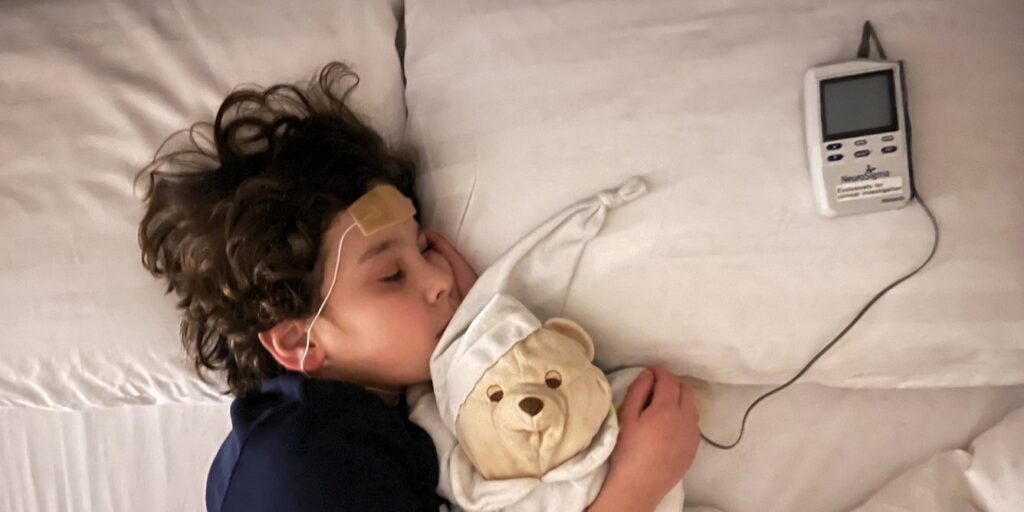
Amid this growing demand, a variety of new treatments are being promoted, some of which are supported by evidence, while others rest on less solid foundations. One such treatment, the trigeminal nerve stimulation (TNS) device, has been marketed as a drug-free option for ADHD. However, a major UK trial has found no evidence that this brain device offers any benefit for children with ADHD.
Understanding ADHD Treatment Options
Stimulant medications like methylphenidate are widely recognized as effective for many children with ADHD, with decades of research supporting their ability to reduce core symptoms and improve functioning at home and school. However, concerns about side effects, stigma, and long-term medication use often lead families to seek alternative treatments.
Brain stimulation devices, such as TNS, have been promoted as a non-medication option. These devices deliver mild electrical stimulation to specific nerves or brain areas and are generally considered safe, with side effects like skin irritation or tingling. However, safety does not equate to effectiveness.
The Rise of Trigeminal Nerve Stimulation
The TNS device, which stimulates the trigeminal nerve—the largest nerve in the face—was cleared by the U.S. Food and Drug Administration (FDA) for ADHD in children in 2019. This clearance, based on a study involving just 62 children, has led to its marketing in private clinics, including in the UK, often at significant costs to families.
The FDA’s decision was based on limited evidence, with the main study showing improvements in ADHD symptoms but having significant weaknesses. Notably, the comparison group received no stimulation, which is crucial as expectations can influence symptom reporting, especially with advanced technology treatments.
New UK Trial Challenges Previous Findings
To address these limitations, a large, independent UK clinical trial was conducted with 150 children and teenagers with ADHD in London and Southampton. The study was designed to control expectations, with participants using identical-looking devices that provided sensations, ensuring neither families nor participants could easily discern if they were receiving real or placebo stimulation.
The findings were clear: there was no evidence that TNS improved ADHD symptoms. Children receiving active stimulation showed no better outcomes than those with the placebo device, with no improvements in attention, behavior, anxiety, mood, or sleep.
“Our findings challenge earlier studies and highlight the importance of large, carefully designed trials, especially for treatments that generate excitement and hope,” researchers noted.
Implications for Families and Future Research
For UK families, the message is significant. While TNS appears safe, effectiveness is crucial. A treatment that does not work offers no real benefit and may divert resources from proven approaches. This study serves as a reminder that regulatory approval or marketing claims do not always indicate effectiveness.
ADHD can be a serious, lifelong condition, and as diagnoses rise, ensuring families receive support and treatments based on robust evidence is vital. The study’s findings underscore the need for rigorous testing to prevent overstating benefits and misleading families.
In response, NeuroSigma, the maker of the TNS device, suggested that the study design might have limited the ability to detect treatment effects, noting the reliance on parent-reported assessments rather than clinician-rated scales. They highlighted an ongoing, larger trial at UCLA, expected to provide further insights later this year.
As the search for effective ADHD treatments continues, this study emphasizes the importance of evidence-based approaches over hype or premature conclusions.





