A groundbreaking study supported by the National Institutes of Health (NIH) has unveiled a potential new avenue for treating neuropathy, a condition known for causing nerve damage. Researchers have discovered that neuropathy disrupts a crucial energy-transfer process between satellite glial cells (SGCs) and the sensory neurons they surround. This discovery could lead to innovative treatments for neuropathy, particularly in conditions like diabetes and chemotherapy-induced nerve damage.
The research team found that mitochondria, the energy-producing machinery of cells, are transferred through tiny tubes between SGCs and neurons. This transfer process was found to be obstructed in animal models of chemotherapy and diabetes. Remarkably, restoring this transfer not only alleviated pain behavior but also promoted nerve regeneration after injury.
Understanding the Energy Transfer Process
The study, led by Ru-Rong Ji, Ph.D., a professor at Duke University School of Medicine, highlights how sensory neurons, which can extend from the spine to the extremities, have a high energy demand. “Sensory neurons can run from near the spine all the way to the tips of your toes and fingers,” Ji explained. “When they fire, they carry signals a great distance, which is why these cells have a particularly high demand for energy.”
While it was previously unclear how these large cells maintained their energy levels, Ji and his colleagues hypothesized that SGCs, known for providing support to sensory neurons, might play a crucial role. Prior research suggested that neural support cells could swap mitochondria, but evidence of this occurring in living organisms was limited.
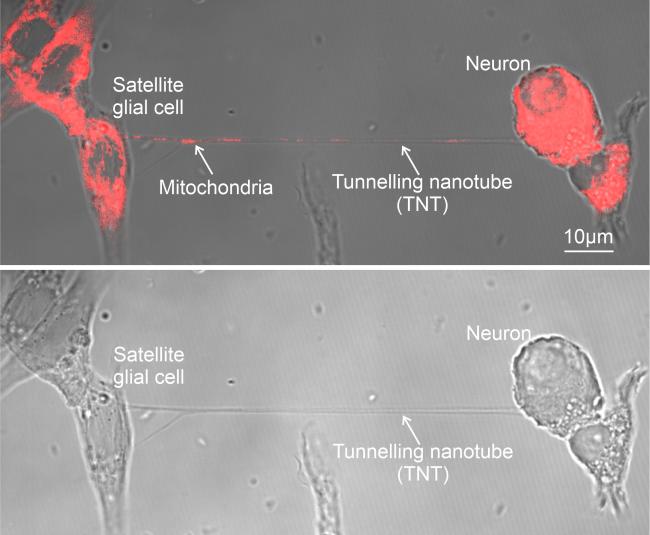
Visualizing Mitochondrial Transfer
To explore this phenomenon, the researchers conducted experiments using mouse cells grown together in a dish. Through high-resolution imaging, they observed the energy supply chain in peripheral neural cells. Further microscopy techniques allowed them to visualize mitochondrial transfer inside tube-like structures in whole dorsal root ganglia from mice.
“The images are quite striking. You can even see bulges in the tubes between cells where the mitochondria are in transit,” Ji noted.
The team confirmed that mitochondria travel through tunneling nanotubes (TNTs) in living mice, and these tubes are essential for regulating pain. They discovered that SGCs primarily initiate tube formation, indicating a one-way transfer from SGCs to neurons.
Implications for Neuropathy Treatment
Delving deeper, the researchers observed how mitochondrial transfer was affected by nerve injury. They found that smaller neurons were the first to lose mitochondria post-injury, with SGCs favoring larger cells, providing them more protection. This vulnerability of smaller neurons to energy loss might explain the prevalence of small fiber neuropathy in chronic conditions.
The study also examined human dorsal root ganglia, revealing that the transfer process is not limited to mice. Tissue comparisons from donors with and without diabetes showed that diabetic SGCs transferred fewer mitochondria to neurons. This suggests that diabetes and certain chemotherapy agents can block mitochondrial transfer, depleting a nerve’s energy reserves and increasing susceptibility to injury.
Exploring Future Avenues
To test if restoring mitochondrial transfer could reverse these effects, researchers induced diabetic or chemotherapy-like conditions in mice. They then transferred healthy human SGCs into the diabetes model and healthy mouse SGCs into the chemotherapy model. Both experiments showed increased pain thresholds in the animals.
Similar results were achieved by isolating mitochondria from SGCs and transferring them into animal models. This not only improved pain thresholds but also potentially restored small nerve branches in the diabetes model.
While these initial results are promising, the researchers acknowledge that much remains to be explored. For instance, they are curious whether astrocytes, a cell type analogous to SGCs, engage in similar mitochondrial transfer processes in the brain and spinal cord.
“There’s only one way to find out,” Ji said. “This and other questions will guide what we examine next.”
This study opens a new chapter in understanding neuropathy and its treatment, offering hope for millions affected by nerve damage worldwide. As research continues, the potential for novel therapies that harness the power of mitochondrial transfer becomes increasingly promising.







