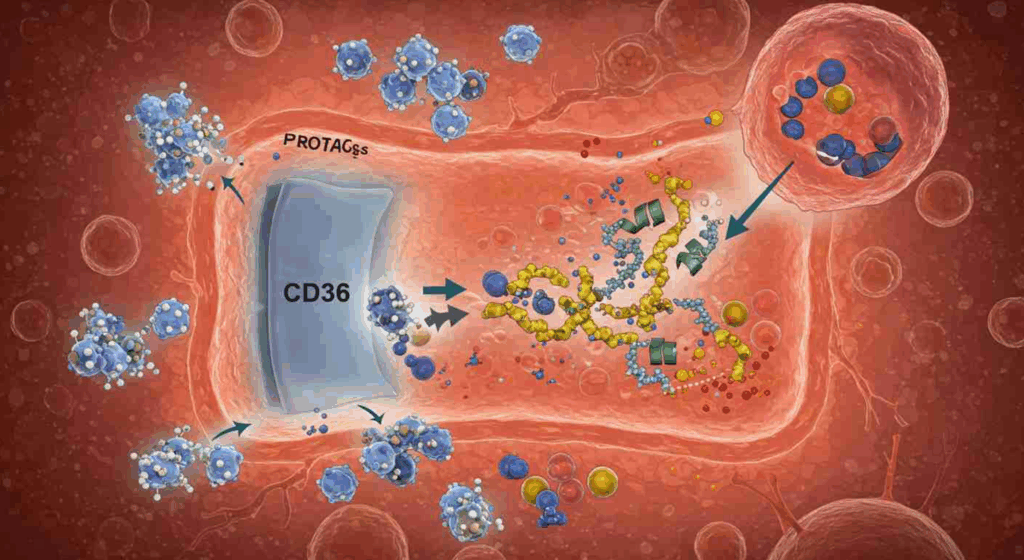
Inside the human body, a little-known protein called CD36 is at the forefront of a groundbreaking discovery that could revolutionize cancer treatment. Scientists have found that CD36 can significantly enhance the delivery of large cancer-fighting drugs into cells, boosting their efficacy by up to 23 times. This breakthrough holds promise for improving the treatment of cancer and other serious diseases by facilitating the entry of complex drugs into cells.
The discovery, made by researchers from Duke University, the University of Texas at San Antonio, and the University of Arkansas, was published in the journal Cell. It reveals that CD36 plays a crucial role in a natural cellular process known as endocytosis, which cells use to absorb nutrients. By leveraging this process, scientists have developed a method to increase the absorption of large cancer drugs, known as PROTACs, by 7.7 to 22.3 times in laboratory tests.
The Science Behind the Breakthrough
Most traditional medications are small molecules designed to pass through the cell membrane easily. However, PROTACs, a new class of drugs, are much larger, often exceeding 1,000 daltons. This size has historically posed a challenge, as larger molecules struggle to penetrate the cell membrane, a concept encapsulated in the “Rule of 5,” which suggests that drugs larger than 500 daltons are generally ineffective at entering cells.
PROTACs work by binding to target proteins and recruiting another protein, E3 ligase, to tag the target for destruction by the cell’s waste-disposal system, the proteasome. This complete degradation of harmful proteins offers a distinct advantage over traditional cancer drugs, which typically only inhibit a single protein function, allowing cancer cells to potentially develop resistance.
CD36: The Gatekeeper
CD36, a protein found on the surface of many cells, including those in the intestines, skin, lungs, and eyes, acts as a gatekeeper, facilitating the entry of large and polar molecules that cannot pass through the cell membrane on their own. By designing PROTACs to attach to CD36, researchers have effectively harnessed this natural process to enhance drug delivery.
“This discovery is important because it could rescue many drugs that were previously considered unusable due to poor absorption,” said Hui-Kuan Lin, PhD, a cancer biology professor involved in the study. “It could turn them into clinically useful treatments.”
Implications for Cancer Treatment and Beyond
This development comes at a time when cancer treatment is rapidly evolving. Unlike older cancer drugs that merely block parts of proteins, PROTACs offer the potential to completely eliminate them, reducing the likelihood of drug resistance. This approach is now being tested for a variety of conditions, including Parkinson’s disease, with eight different oral PROTACs currently in clinical trials.
Researchers have also explored the interaction between PROTACs and CD36 using advanced techniques like surface plasmon resonance. These studies revealed that modified PROTACs, such as SIM1-Me, exhibit stronger binding to CD36 than natural molecules like palmitic acid, further enhancing their cellular uptake and efficacy.
Potential Beyond Cancer
The implications of this discovery extend beyond cancer treatment. Many diseases involve proteins previously deemed “undruggable” due to their size or complexity. The chemical endocytic medicinal chemistry (CEMC) strategy, which focuses on utilizing proteins like CD36 for drug delivery, could pave the way for new treatments for conditions such as Alzheimer’s, autoimmune disorders, and infectious diseases.
“This was completely unexpected in the research field,” said Hong-yu Li, PhD, a medicinal chemistry professor at UT San Antonio. “Through chemistry and biology, we identified CD36 as a protein for uptake and optimized drugs to better engage with CD36.”
Next Steps and Future Research
While the results are promising, the next step involves testing this method in human clinical trials to ensure its safety and efficacy in real patients. The early data is hopeful, suggesting a potential shift in how we approach drug design and disease treatment.
This discovery represents more than just a clever chemical trick; it signifies a paradigm shift in medical research. For decades, scientists have focused on designing drugs to bypass the cell membrane. Now, with the help of CD36, they may have found a more efficient pathway to deliver life-saving treatments.
The research, confirmed independently by all three collaborating teams, underscores the potential of CD36-mediated endocytosis as a general pathway for the uptake of large therapeutic molecules. As the scientific community continues to explore this avenue, the hope is that it will lead to new, more effective therapies for a range of diseases.




