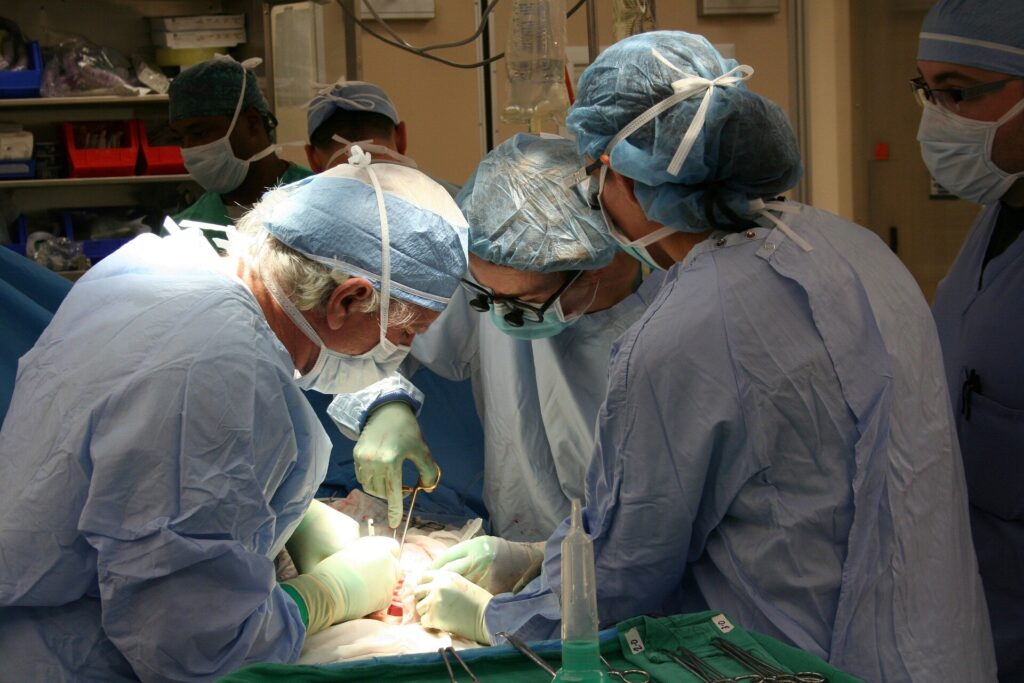
In a Maryland operating room in November 2025, doctors achieved a groundbreaking medical feat by transplanting a genetically modified pig kidney into a living patient. This kidney, engineered to mimic human tissue, was grown in a pig as an alternative to the often interminable wait for a human organ donor. Once a concept relegated to the realm of science fiction, this procedure is now a tangible reality.
The patient is part of a pioneering clinical trial involving six individuals testing the viability of pig-to-human kidney transplants. The primary aim is to determine whether these gene-edited pig kidneys can safely function as replacements for failing human kidneys.
From Science Fiction to Medical Reality
A decade ago, scientific efforts were directed towards a different solution. Researchers aimed to grow human organs entirely from human cells within pigs. However, in 2015, the National Institutes of Health (NIH) halted funding for this research due to ethical concerns, a pause that persists today.
As a bioethicist who has extensively studied the ethics of using animal-grown organs, I find this decision perplexing. The ban was based on fears of making pigs too human-like, yet regulators now seem comfortable with the reverse—making humans a bit more pig-like.
The Urgent Need for Xenotransplantation
The desperation driving these experiments is often overlooked. Over 100,000 Americans are on organ transplant waiting lists, with demand far outstripping supply. Thousands die annually before a suitable organ becomes available.
For decades, scientists have explored interspecies solutions, from baboon hearts in the 1960s to today’s genetically altered pigs. The primary challenge has always been the immune system, which treats foreign cells as invaders and destroys them.
More than 100,000 Americans are waiting for organ transplants, highlighting a critical shortage.
A recent case in New Hampshire involved a man who received a gene-edited pig kidney in January 2025. Although initially successful, the kidney had to be removed nine months later due to declining function. This partial success underscores the ongoing challenge of transplant rejection, a central issue in xenotransplantation.
Alternative Approaches and Their Challenges
Researchers are attempting to overcome transplant rejection by creating organs that the human body might accept, involving the insertion of human genes and the deletion of certain pig genes. Despite these efforts, recipients of gene-edited pig organs require powerful immunosuppressants, which may not fully prevent rejection.
Another promising approach involved growing organs from a patient’s own cells. This method entailed disabling genes in pig embryos responsible for organ development and injecting human stem cells to fill the gap. Theoretically, this would result in a kidney genetically matched to a future patient, eliminating rejection risks.
Despite its promise, this method is technically complex due to the differing developmental speeds of human and pig cells. However, researchers had already demonstrated feasibility by growing a mouse pancreas inside a rat, proving cross-species organ growth was not merely a fantasy.
Ethical Concerns and the NIH Ban
The NIH’s 2015 ban on inserting human stem cells into animal embryos was driven not by scientific failure but by moral confusion. Policymakers feared that human cells might integrate into the animal’s body, particularly the brain, potentially blurring the line between human and animal.
The NIH cautioned against possible “alterations of the animal’s cognitive state.” The Animal Legal Defense Fund argued that if such chimeras gained humanlike awareness, they should be treated as human research subjects.
“The NIH warned of possible ‘alterations of the animal’s cognitive state,’ raising ethical concerns.”
The core concern is whether an animal’s moral status might change, necessitating better treatment due to increased vulnerability to harm. Sentient animals, capable of experiencing sensations like pain, differ from self-conscious animals, which can reflect on their experiences, leading to deeper harm.
Reevaluating the NIH’s Stance
The NIH’s reasoning appears flawed. If cognitive capacities like self-consciousness conferred higher moral status, regulators would be equally concerned about inserting dolphin or primate cells into pigs as they are about human cells. They are not.
In practice, moral concern is often based on species membership rather than cognitive abilities. All humans are protected from harmful research due to their humanity, not specific cognitive capacities. No research objective can justify violating basic human interests.
If a pig embryo with human cells became sufficiently human-like, it would warrant human-level regard under current research regulations. However, the mere presence of human cells doesn’t transform pigs into humans.
“The pigs engineered for kidney transplants already carry human genes, but they aren’t called half-human beings.”
While there may be valid objections to using animals as organ factories, the rationale behind the NIH ban—that human cells could make pigs too human—rests on a misunderstanding of what grants beings moral standing.
Looking Forward: Balancing Ethics and Innovation
The ethical landscape of xenotransplantation is complex and evolving. As science advances, so too must our ethical frameworks. Balancing the urgent need for organ transplants with ethical considerations will require ongoing dialogue among scientists, ethicists, and policymakers.
As researchers continue to push the boundaries of what’s possible, society must grapple with these ethical dilemmas, ensuring that scientific progress does not outpace our moral compass.