Biomedical researchers at Texas A&M University have unveiled a groundbreaking technique that could potentially halt or even reverse the loss of cellular energy associated with damage and aging. This innovative discovery, if validated by future studies, promises to transform the treatment of numerous diseases across the medical field.
Dr. Akhilesh K. Gaharwar and Ph.D. student John Soukar, along with their team in the Department of Biomedical Engineering, have developed a method to supply injured cells with fresh mitochondria. By replenishing these vital energy producers, the technique can restore energy output to previous levels, significantly enhancing the overall health of the cells.
Understanding Mitochondrial Decline and Its Impact
Mitochondrial decline is a well-documented phenomenon linked to aging, heart disease, and several neurodegenerative conditions. A strategy that bolsters the body’s natural ability to replace worn-out mitochondria could potentially address these issues simultaneously.
As human cells age or suffer damage from degenerative disorders like Alzheimer’s disease or from exposure to harmful agents such as chemotherapy drugs, their energy generation capacity diminishes. This decline is primarily due to the decreasing number of mitochondria, the small organelles within cells responsible for most of the cell’s energy production. Whether in brain tissue, heart muscle, or other organs, a reduction in mitochondria results in weaker, less healthy cells that eventually lose their ability to perform essential functions.
Nanoflowers: A New Tool for Stem Cell Enhancement
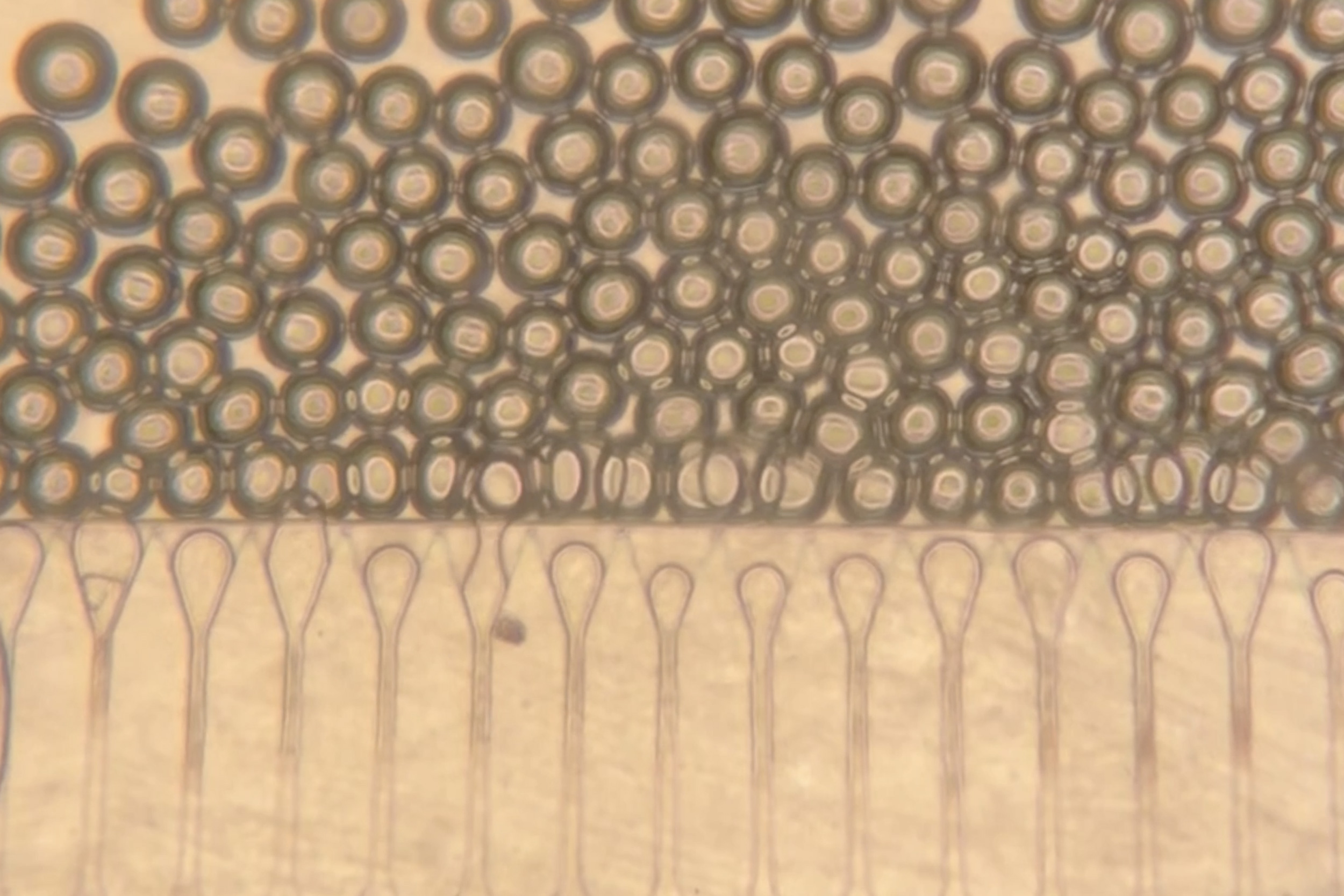
The research, published in the Proceedings of the National Academy of Sciences, introduces the use of microscopic, flower-shaped particles known as nanoflowers in conjunction with stem cells. When stem cells are exposed to these nanoflowers, they begin producing approximately twice as many mitochondria as usual. These enhanced stem cells, when placed next to damaged or aging cells, transfer their surplus mitochondria to the injured neighbors.
Once endowed with new mitochondria, the previously damaged cells can restore their energy production and resume normal activity. These rejuvenated cells not only exhibit improved energy levels but also demonstrate increased resistance to cell death, even when subjected to damaging treatments like chemotherapy.
“We have trained healthy cells to share their spare batteries with weaker ones,” said Gaharwar, a professor of biomedical engineering. “By increasing the number of mitochondria inside donor cells, we can help aging or damaged cells regain their vitality — without any genetic modification or drugs.”
Efficiency and Potential of Mitochondrial Bio Factories
Although cells naturally exchange small amounts of mitochondria, the nanoflower-treated stem cells, described by the team as mitochondrial bio factories, transfer two to four times more mitochondria than untreated stem cells.
“The several-fold increase in efficiency was more than we could have hoped for,” said Soukar, lead author of the paper. “It’s like giving an old electronic a new battery pack. Instead of tossing them out, we are plugging fully-charged batteries from healthy cells into diseased ones.”
Advancing Mitochondria-Based Therapies
Researchers have explored various methods to increase mitochondrial numbers within cells, but these often come with limitations. Drug-based approaches rely on small molecules that are quickly metabolized, necessitating frequent treatments. In contrast, the larger nanoparticles, approximately 100 nanometers in diameter, remain inside the cell longer and stimulate more effective mitochondria production. Consequently, therapies utilizing this nanoflower technology might only require monthly administration.
“This is an early but exciting step toward recharging aging tissues using their own biological machinery,” Gaharwar said. “If we can safely boost this natural power-sharing system, it could one day help slow or even reverse some effects of cellular aging.”
The Role of Molybdenum Disulfide Nanoparticles
The nanoflowers are crafted from molybdenum disulfide, an inorganic compound capable of forming various two-dimensional shapes at microscopic scales. The Gaharwar Lab is among the few research groups exploring the biomedical applications of molybdenum disulfide.
Stem cells already play a pivotal role in cutting-edge tissue repair and regeneration research. Enhancing stem cell performance with nanoflowers could represent a significant advancement in making these cells even more effective in future therapies.
Versatility and Future Applications
One of the most promising aspects of this technique is its versatility. Although still in its infancy and requiring extensive testing, the method could theoretically be applied to treat loss of function in various tissues throughout the body.
“You could put the cells anywhere in the patient,” Soukar said. “So for cardiomyopathy, you can treat cardiac cells directly — putting the stem cells directly in or near the heart. If you have muscular dystrophy, you can inject them right into the muscle. It’s pretty promising in terms of being able to be used for a whole wide variety of cases, and this is just kind of the start. We could work on this forever and find new things and new disease treatments every day.”
The project has garnered financial support from prestigious institutions, including the National Institutes of Health, the Welch Foundation, the Department of Defense, and the Cancer Prevention and Research Institute of Texas. Additional backing came from the President’s Excellence Fund at Texas A&M University and the Texas A&M Health Science Center Seedling Grant. Key collaborators include Texas A&M researchers Dr. Irtisha Singh, Dr. Vishal Gohil, and Dr. Feng Zhao.