Statins have revolutionized heart health by significantly lowering cholesterol levels and reducing the risk of heart attacks and strokes. However, for many patients, these life-saving drugs come with a troubling side effect: muscle pain, weakness, and in rare cases, severe muscle breakdown that can lead to kidney failure. Researchers at the University of British Columbia (UBC), in collaboration with the University of Wisconsin-Madison, have identified the cause of these adverse effects, offering hope for a new generation of statins without such drawbacks.
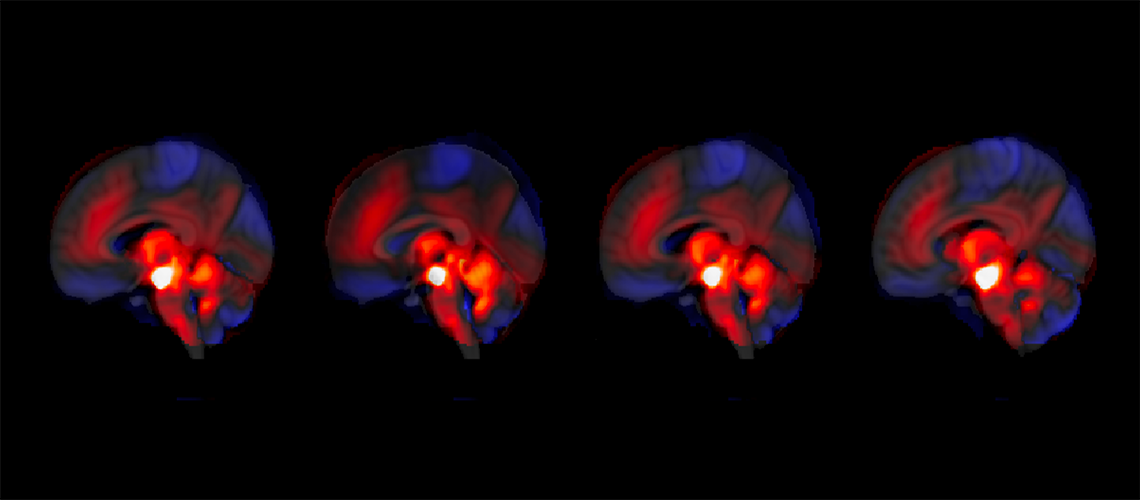
Their groundbreaking findings, published last week in Nature Communications, could pave the way for safer statins. The research team utilized cryo-electron microscopy, a powerful imaging technique that reveals proteins at near-atomic detail, to observe how statins interact with a critical muscle protein known as the ryanodine receptor (RyR1). This protein functions as a gatekeeper for calcium inside muscle cells, opening only when muscles need to contract. Statins, as it turns out, bind to this receptor and force the gate open, causing a continuous leak of calcium—a toxic effect that can damage muscle tissue.
Insights from Advanced Imaging Techniques
“We were able to see, almost atom by atom, how statins latch onto this channel,” explained lead author Dr. Steven Molinarolo, a postdoctoral researcher in UBC’s department of biochemistry and molecular biology. “That leak of calcium explains why some patients experience muscle pain or, in extreme cases, life-threatening complications.”
The study focused on atorvastatin, one of the most widely prescribed statins, but the findings suggest that this effect may be common across the entire class of drugs. The researchers discovered that statins bind in a highly unusual manner: three molecules cluster together inside a pocket of the protein. The first molecule attaches when the channel is closed, priming it to open, while two additional molecules wedge in, forcing the channel wide open.
Designing Safer Statins
“This is the first time we’ve had a clear picture of how statins activate this channel,” stated Dr. Filip Van Petegem, senior author and professor at UBC’s Life Sciences Institute. “It’s a big step forward because it gives us a roadmap for designing statins that don’t interact with muscle tissue.”
By modifying only those parts of the statin molecule responsible for the negative effects, scientists could potentially preserve the cholesterol-lowering benefits while minimizing the risk of muscle damage. Although severe muscle damage affects only a small fraction of the over 200 million statin users worldwide, milder symptoms like aches and fatigue are more common and often lead patients to discontinue treatment. The new findings could help prevent these issues and improve adherence to life-saving therapy.
The Role of Advanced Technology in Medical Breakthroughs
The research underscores the importance of advanced imaging technology in driving medical breakthroughs. Using UBC’s high-resolution macromolecular cryo-electron microscopy facility, the team visualized the statin-protein interaction in extraordinary detail, transforming a fundamental question about drug safety into practical insights that could shape the next generation of therapies.
“Statins have been a cornerstone of cardiovascular care for decades,” Dr. Van Petegem said. “Our goal is to make them even safer, so patients can benefit without fear of serious side effects.”
For millions of people who rely on statins, this could mean fewer muscle-related problems and an improved quality of life. As the research progresses, the hope is that these findings will lead to the development of statins that maintain their efficacy in lowering cholesterol without the associated muscle risks.
Meanwhile, the medical community continues to explore these promising avenues, aiming to enhance patient care and safety. The potential for a new generation of statins represents a significant advancement in cardiovascular treatment, offering renewed hope for patients worldwide.