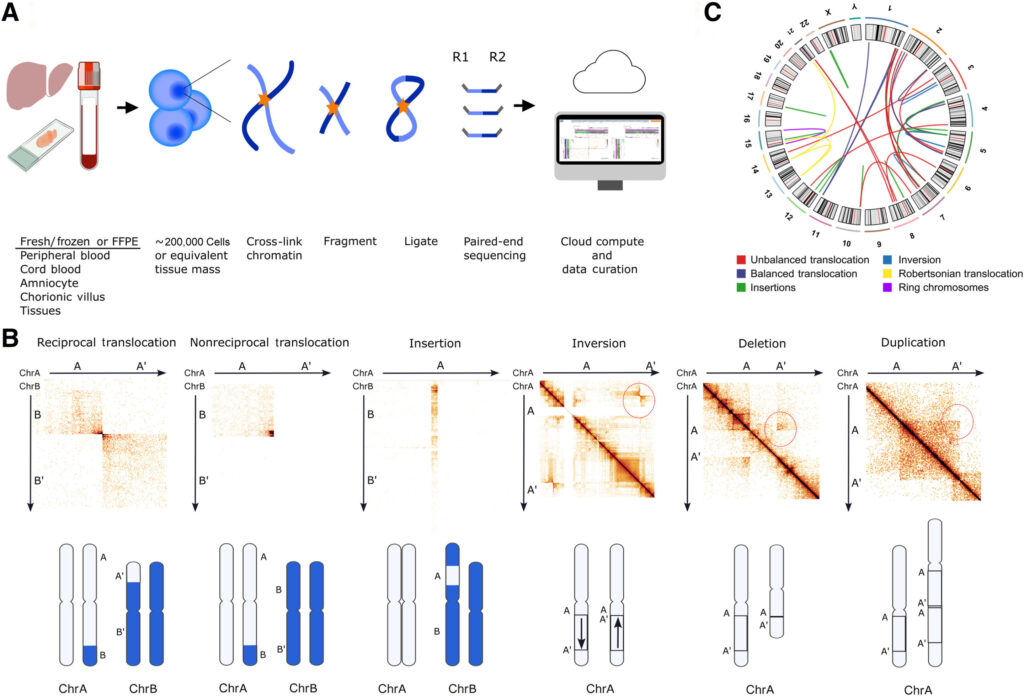
Standard laboratory tests often fail to detect numerous disease-causing DNA changes. Now, a groundbreaking 3D chromosome mapping method has emerged, capable of reliably revealing these hidden structural variants, potentially leading to new discoveries in genetic research. A study published in The Journal of Molecular Diagnostics highlights this innovative method, which is set to revolutionize diagnostic testing and treatment for genetic disorders.
Traditional DNA sequencing methods have long been constrained by their linear, one-dimensional approach, treating the genetic code as a flat line of text. In stark contrast, 3D chromosome mapping captures the spatial relationships between different genome parts, revealing how DNA strands fold and interact within the cell nucleus. This spatial awareness is crucial for detecting structural changes that remain invisible to conventional linear tests.
Breakthrough in Genomic Analysis
Researchers have applied genomic proximity mapping (GPM), a genome-wide Hi-C (high-throughput chromosome conformation capture sequencing)-based NGS assay, to DNA samples from 123 individuals with suspected genetic disorders. This approach captured the 3D contacts within the genome, enabling the detection of both copy-number changes and DNA rearrangements. Remarkably, GPM correctly identified all known large chromosomal variants, including 110 deletions/duplications and 27 rearrangements, achieving 100% concordance. Moreover, it uncovered 12 novel structural variants that standard clinical tests missed.
“We were excited by how much hidden complexity GPM revealed,” said co-lead investigator He Fang, Ph.D., from the Department of Laboratory Medicine and Pathology at the University of Washington School of Medicine, Seattle.
Dr. Fang further explained, “Using modern tools like GPM allows us to uncover hidden DNA rearrangements that standard tests miss. For instance, one case with a known three-way translocation actually had 13 breakpoints across four chromosomes when mapped by GPM. In every patient with multiple rearrangements, GPM uncovered additional cryptic changes.”
Key Findings and Implications
- 100% Detection of Known Variants: GPM identified all 110 previously recognized copy-number variants and 27 rearrangements in the cohort.
- High Precision on Complex Events: Breakpoints of both balanced and unbalanced rearrangements were pinpointed with high accuracy (within ~10 kb), even in challenging samples like preserved tissue, while detecting mosaic changes.
- New Discoveries: GPM revealed 12 additional structural variants that standard methods had missed.
- Hidden Complexity: In every case with multiple rearrangements by traditional tests, GPM found extra cryptic changes.
GPM requires significantly less DNA than conventional cytogenetic methods or emerging technologies such as optical genome mapping (OGM) and long-read sequencing (LRS), enhancing its practicality for real-world clinical implementation. Identifying the exact genetic rearrangement could open doors to targeted therapies or clinical trials specific to those variants.
Transforming Genetic Diagnostics
Co-lead investigator Yajuan J. Liu, Ph.D., also from the Department of Laboratory Medicine and Pathology at the University of Washington School of Medicine, Seattle, emphasized the broad clinical benefits of GPM. “It enables high-resolution, comprehensive genomic characterization, even from compromised samples such as low-quality or archived preserved tissue,” Dr. Liu noted.
“As genomic medicine moves toward precision diagnostics, this new tool addresses current limitations in genetic testing, improving diagnostics and empowering doctors to provide personalized treatment, tailored monitoring, better prognosis, and improved family counseling.”
This development follows a growing trend in genomic medicine towards precision diagnostics. By addressing current limitations in genetic testing, GPM not only improves diagnostic accuracy but also empowers healthcare providers to offer personalized treatment, tailored monitoring, and better prognostic assessments.
Looking Ahead
The introduction of 3D genome mapping represents a significant leap forward in genetic research and diagnostics. As the method gains traction, it promises to enhance our understanding of genetic disorders and pave the way for more effective treatments. The implications of this technology extend beyond individual patient care, potentially influencing broader medical practices and research methodologies.
As researchers continue to explore the full potential of 3D genome mapping, the future of genetic diagnostics looks promising, with the potential for more precise, personalized, and effective healthcare solutions.







