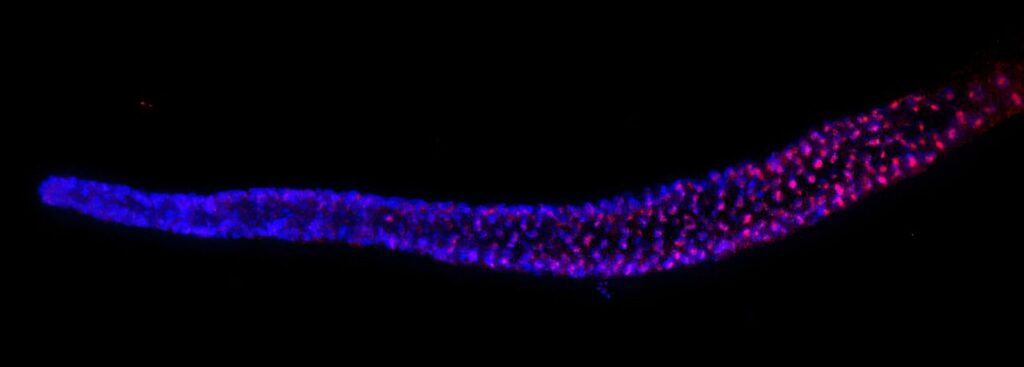
An international research team led by Hiroki Shibuya at the RIKEN Center for Biosystems Dynamics Research in Japan has uncovered a groundbreaking genetic mechanism that may hold the key to preventing species extinction and advancing anti-aging therapies. Published in the journal Science, the study reveals that in the roundworm C. elegans, essential RNA for chromosome protection hitchhikes within another gene, a discovery that could have far-reaching implications for regenerative medicine and understanding cellular aging.
The research focuses on telomeres, the protective DNA caps at the ends of chromosomes, which function much like the plastic tips of shoelaces. As somatic cells divide to regenerate tissue, these telomeres gradually shorten, contributing to aging and cellular self-destruction when they become too short. However, germ cells—precursors to sperm and egg cells—maintain their telomere length through the enzyme telomerase, ensuring species survival across generations.
The Genetic Mystery of Telomerase RNA
Telomerase relies on an RNA template to replenish telomeres. In mammals, this RNA is produced by the TERC gene. However, C. elegans possesses functional telomerase without a discernible TERC gene, a conundrum that has puzzled scientists for over two decades. The RIKEN research team has now identified that in C. elegans, the necessary RNA, dubbed terc-1, is embedded within an intron of the gene nmy-2, which is active only in germ cells.
Introns are typically non-coding segments within genes that are removed after the gene’s protein is synthesized. The discovery that terc-1 is hidden within an intron was unexpected, as explained by Shibuya:
“It was surprising to find that the key RNA—which we have named terc-1—was hidden inside an intron of the gene called nmy-2, which is expressed only in germ cells.”
Implications for Species Survival and Medicine
Experiments demonstrated that C. elegans lacking terc-1 experienced progressive telomere shortening, leading to extinction within 15 generations. Conversely, inserting terc-1 into introns of other germ-cell-expressed genes restored normal telomere length and species viability. This suggests that terc-1’s strategic placement ensures its expression where needed, safeguarding future generations from telomere-related extinction.
The research team believes this intron hitchhiking strategy is not unique to C. elegans. Shibuya notes,
“Although we discovered this intron hitchhiking strategy in C. elegans, similar mechanisms are likely used by other non-coding RNAs or exist across different species.”
Broader Biological and Therapeutic Insights
This discovery not only sheds light on evolutionary biology but also offers potential pathways for medical advancements. Understanding telomerase regulation in healthy cells could revolutionize treatments for aging, fertility, and regenerative medicine. The concept of embedding RNAs to control their expression timing and location may represent a broader biological principle.
As researchers continue to explore this phenomenon, the implications for human health and longevity remain profound. The ability to manipulate telomerase activity and telomere maintenance could pave the way for novel therapies aimed at extending healthy lifespan and combating age-related diseases.
Moving forward, the scientific community will likely investigate whether similar genetic strategies are employed by other organisms and how these insights can be translated into practical medical applications. The discovery of DNA hitchhiking within genes marks a significant step in our understanding of genetic regulation and its potential to transform human health.