MIT chemists have unveiled a groundbreaking fluorescent molecule that holds promise for significantly improving biomedical imaging, particularly in generating clearer images of tumors. This innovative dye is based on a borenium ion—a positively charged form of boron—capable of emitting light in the red to near-infrared spectrum. Historically, the instability of these ions has hindered their application in imaging and other biomedical fields.
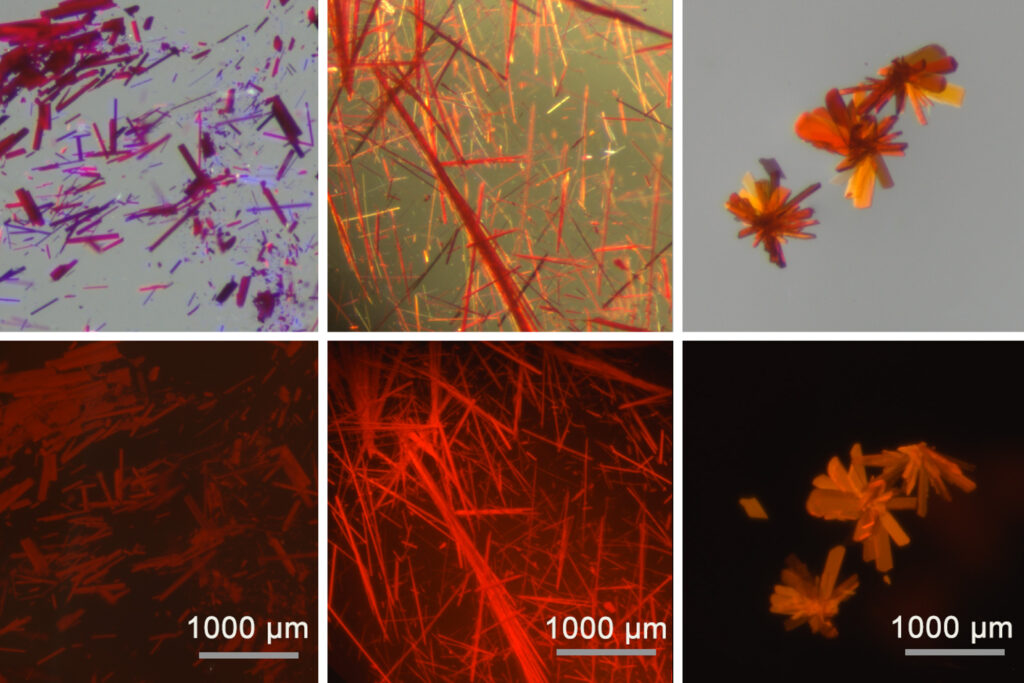
In a study published today in Nature Chemistry, the researchers demonstrated that attaching borenium ions to a ligand can stabilize them, resulting in borenium-containing films, powders, and crystals. These materials emit and absorb light in the red and near-infrared range, which is crucial for imaging deep tissue structures, potentially offering clearer images of tumors and other bodily structures.
Stabilizing Borenium Ions
The development of this new dye addresses a significant challenge in fluorescent imaging, which traditionally relies on dyes emitting blue or green light. These dyes function well at the cellular level but are less effective in tissues due to interference from the body’s natural fluorescence and the scattering of blue and green light, which limits penetration depth.
Red fluorescent dyes can produce clearer images, but they often suffer from instability and low brightness due to poor quantum yields. Borenium cations, discovered in the mid-1980s, were initially considered “laboratory curiosities” due to their extreme instability, requiring handling in sealed environments to prevent breakdown from air exposure.
According to Robert Gilliard, the Novartis Professor of Chemistry at MIT and senior author of the study, “One of the reasons why we focus on red to near-IR is because those types of dyes penetrate the body and tissue much better than light in the UV and visible range. Stability and brightness of those red dyes are the challenges that we tried to overcome in this study.”
Innovative Applications and Future Directions
By attaching borenium ions to molecules known as ligands, Gilliard’s lab discovered in 2019 that these ions could change emission colors with temperature variations. However, they remained too reactive for open-air handling. The breakthrough came with the use of carbodicarbenes (CDCs) to further stabilize these ions, allowing them to be studied without a glovebox and making them resistant to light-induced degradation.
In the latest study, Gilliard’s team explored interactions between the anions and borenium cations in CDC-borenium compounds, discovering exciton coupling. This phenomenon shifted the emission and absorption properties towards the infrared spectrum and increased the quantum yield significantly.
“Not only are we in the correct region, but the efficiency of the molecules is also very suitable,” Gilliard notes. “We’re up to percentages in the thirties for the quantum yields in the red region, which is considered to be high for that region of the electromagnetic spectrum.”
Potential Biomedical and Technological Uses
The researchers have shown that these compounds can be transformed into various states, including solid crystals, films, powders, and colloidal suspensions. For biomedical imaging, the vision is to encapsulate these materials in polymers for injection as imaging dyes. Initial collaborations with MIT and the Broad Institute aim to explore cellular imaging capabilities.
These materials’ temperature responsiveness also suggests potential as temperature sensors, useful for monitoring the thermal exposure of drugs or vaccines during transportation. Gilliard highlights their utility as “molecular thermometers” for any application where temperature tracking is crucial.
Frieder Jaekle, a professor of chemistry at Rutgers University, who was not involved in the study, emphasizes the compounds’ potential: “The very high quantum yields achieved in the near-IR, combined with the excellent environmental stability, make this class of compounds extremely interesting for biological applications. Besides the obvious utility in bioimaging, the strong and tunable near-IR emission also makes these new fluorophores very appealing as smart materials for anticounterfeiting, sensors, switches, and advanced optoelectronic devices.”
Looking Ahead
Beyond current applications, the research team is working on extending the color emission further into the near-infrared region by incorporating additional boron atoms. This could enhance the dyes’ capabilities but may also affect stability, prompting the development of new carbodicarbenes to maintain stability.
The research, funded by the Arnold and Mabel Beckman Foundation and the National Institutes of Health, marks a significant step forward in the field of biomedical imaging, potentially transforming how medical professionals visualize and diagnose conditions deep within the human body.