In a groundbreaking advancement for medicine, researchers at the University of Washington have developed programmable proteins capable of improving targeted drug delivery. Published on October 9 in Nature Chemical Biology, the study demonstrates how these proteins, equipped with autonomous decision-making capabilities, can enhance the precision of therapies by responding to multiple biomarkers.
This development holds significant promise for the field of targeted drug delivery, which aims to direct therapies specifically to the areas of the body where they are needed, thereby minimizing dosage and reducing harmful side effects. For instance, targeted immunotherapies could activate immune cells to combat cancerous tissues exclusively, sparing healthy cells from unnecessary exposure.
Innovative Approach to Drug Delivery
The challenge in creating “smart” therapies lies in enabling medicines to navigate the body and identify their precise targets. The University of Washington team has made strides in this direction by designing proteins with smart tail structures that fold into preprogrammed shapes. These structures allow the proteins to localize based on specific environmental cues, effectively acting as logical circuits that determine the proteins’ actions.
Senior author Cole DeForest, a professor of chemical engineering and bioengineering at UW, expressed excitement about the potential of these systems. “We’ve now finally figured out how to produce these systems faster, at scale, and with dramatically enhanced logical complexity,” DeForest stated. “We are excited about how these will lead to more sophisticated and scalable disease-honing therapies.”
Building on Established Concepts
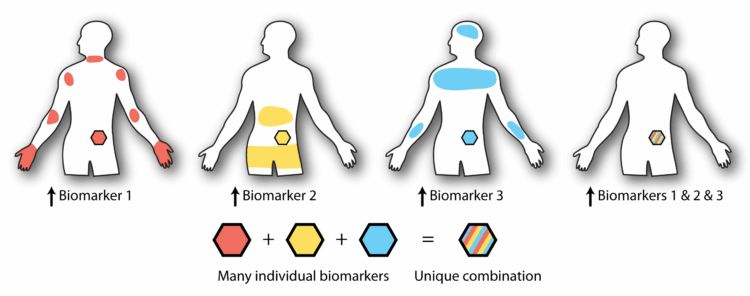
The idea of programmable biomaterials is not entirely new. Scientists have long sought to make systems responsive to individual cues, such as pH levels or specific enzymes. However, finding a unique biomarker for a single location in the body is rare, leading researchers to explore combinations of biomarkers for more precise targeting.
In 2018, DeForest’s lab pioneered materials that responded to multiple biomarkers using Boolean logic, a concept borrowed from computer science. This approach allowed the creation of logical circuits that could perform complex operations based on the presence of multiple biomarkers.
“We realized that we could program how therapeutics were released based simply on how they were connected to a carrier material,” DeForest explained. “By combining basic gates, we could readily create advanced logical circuits.”
Advancements in Synthetic Biology
The field of synthetic biology has seen rapid advancements, enabling the production of complex proteins more efficiently. Co-first author Murial Ross, a doctoral student of bioengineering at UW, highlighted the impact of these tools. “The field has developed exciting new protein-based tools that can allow researchers to form permanent bonds between proteins,” Ross noted. “It opened doors for new protein structures that were previously unachievable.”
With these advancements, the research team was able to streamline the production process, designing proteins that spontaneously fold into bespoke shapes. These proteins can respond to up to five different biomarkers and attach to various carriers for delivery to targeted sites within the body.
Future Implications and Applications
The implications of this research are vast, with potential applications extending beyond cancer treatments. The technology could also be used to manufacture therapies within a single cell, directing them to specific regions, or even develop diagnostic tools like blood tests that react to complex cues.
DeForest envisions a future where materials can be programmed to act at any arbitrary location inside the body. “That’s a tall order, but with these technologies, we’re getting closer,” he said. “With the right combination of biomarkers, these materials will just get more and more precise.”
The research, funded by the National Science Foundation and the National Institutes of Health, involved contributions from several UW students and alumni, including Ryan Gharios, Annabella Li, Shivani Kottantharayil, and Jack Hoye.
As the team continues to explore new biomarkers and collaborates with other laboratories, the potential for real-world therapies becomes increasingly tangible. The advancements in programmable proteins mark a significant leap forward in the quest for more effective and precise medical treatments.