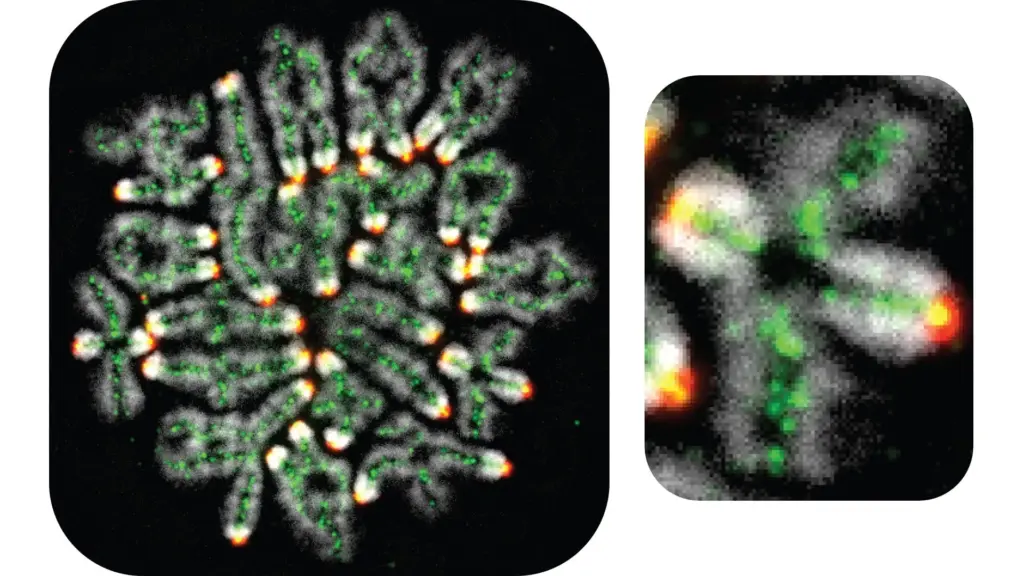
“If that goes wrong, then you end up with the wrong number of chromosomes in the eggs or sperm,” said Neil Hunter, a professor in the Department of Microbiology and Molecular Genetics at the University of California, Davis. “This can result in infertility, miscarriage or the birth of children with genetic diseases.”
In a groundbreaking study published on September 24 in the journal Nature, Hunter’s team unveiled a significant discovery regarding a DNA process that helps prevent these chromosomal errors. The research provides a detailed look at the choreography of proteins that ensure the correct sorting of chromosomes as egg and sperm cells develop and divide.
Understanding Chromosome Crossovers
Humans possess 46 chromosomes in each cell, organized into 23 pairs of homologous chromosomes. Early in the development of sperm or egg cells, these chromosome pairs align, allowing the parental chromosomes to break and rejoin, a process known as “crossovers.” These crossovers serve two critical functions: they ensure genetic diversity by mixing genes from both parents and maintain connections between chromosome pairs, guiding their distribution during cell division.
According to Hunter, maintaining crossover connections is particularly crucial in females. During the development of egg cells, these connections are formed and then held in a state of suspended animation until ovulation, sometimes for decades. “Maintaining the crossover connections over many years is a major challenge for immature egg cells,” Hunter noted.
“If chromosome pairs aren’t connected by at least one crossover, they can lose contact with each other, like two people separated in a jostling crowd.”
This disconnection can lead to incorrect segregation during cell division, resulting in egg cells with extra or missing chromosomes. Such errors can cause infertility, miscarriages, or genetic conditions like Down syndrome, characterized by an extra copy of chromosome 21.
From Yeast to Human Implications
Hunter’s research, conducted using budding yeast, a model organism, has provided unprecedented insights into the molecular events of chromosome recombination. By employing genetic engineering techniques, the team was able to observe these processes with remarkable detail. “The chromosome structures that we studied have changed very little across evolution,” Hunter explained, emphasizing the relevance of these findings to human biology.
The research identified dozens of proteins involved in the formation of double-Holliday junctions, critical structures in crossover formation. Using “real-time genetics,” Hunter and his team could manipulate and observe these proteins’ roles, revealing a complex network that ensures crossovers are properly formed. Key among these proteins is cohesin, which prevents premature dismantling of the junctions by an enzyme complex known as STR in yeast, or Bloom in humans.
“They protect the double Holliday junction,” Hunter said. “That is a key discovery.”
Implications for Fertility Treatments
This discovery has significant implications for understanding and potentially treating fertility issues in humans. The failure to protect double-Holliday junctions may be a contributing factor to reproductive problems. The research, supported by the National Institutes of Health and other prestigious institutions, highlights the potential for new diagnostic and therapeutic strategies in human fertility.
The study also underscores the collaborative efforts of the scientific community, with contributions from postdoctoral researchers and undergraduate students at UC Davis. The work utilized advanced facilities at the university, including the Proteomics Core Facility and the Comprehensive Cancer Center.
As researchers continue to unravel the complexities of chromosome behavior, Hunter’s findings pave the way for future breakthroughs in genetics and fertility. The study not only enhances our understanding of fundamental biological processes but also offers hope for addressing some of the most challenging reproductive issues faced today.