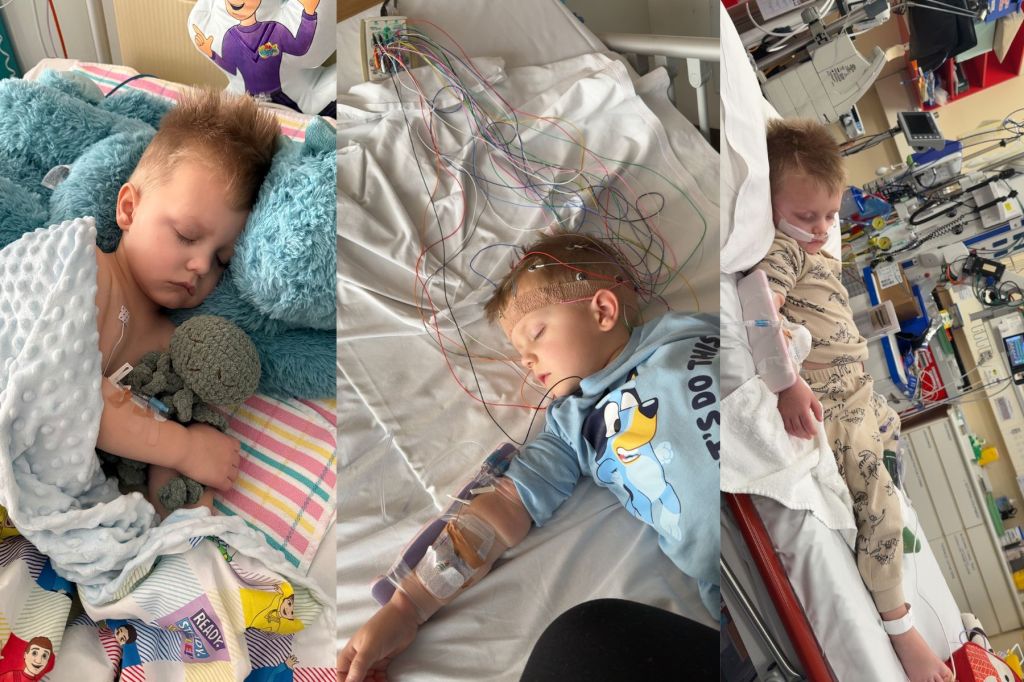
Three-year-old Clive Bussenschutt from South Australia is currently undergoing rehabilitation after suffering severe side effects from influenza, including brain inflammation. Clive’s condition developed after he missed receiving a flu vaccine this year. In response to cases like Clive’s, the South Australian government has announced the rollout of a new needle-free flu vaccine for children, aiming to prevent similar hospitalizations in the future.
Health Minister Chris Picton revealed that the new nasal spray vaccine, FluMist, will be available free of charge for children aged between two and five starting next year. This initiative is part of a broader strategy to improve vaccination rates among young children, a group particularly vulnerable to severe flu complications.
Impact of Flu on Young Children
Clive’s mother, Monique Bussenschutt, expressed her support for the new vaccine, stating, “If FluMist was an option, we would have 100 percent gone for it.” She recounted how Clive fell ill rapidly, experiencing severe symptoms such as high fevers, seizures, and low oxygen levels. “Clive has now been diagnosed with a permanent brain injury. He’s still in rehab at the moment, trying to get back to his baseline,” she added.
The announcement comes as the state grapples with the impact of influenza on young children. According to Health Minister Picton, 1,806 young children were affected by the flu this year, with more than 178 requiring hospitalization due to severe side effects. He emphasized the importance of targeting young children in public health campaigns, noting that only about 27 percent of young kids are currently vaccinated.
FluMist: A Game-Changer in Vaccination
Minister Picton described FluMist as a “game-changing” product that could significantly increase vaccination rates. “Vaccines can be scary for kids. Our doctors and nurses do incredible work to make it as easy as possible for kids to get vaccinated. But this FluMist has the potential to make it even easier,” he stated.
FluMist, produced by AstraZeneca, will be administered by general practitioners in South Australia. Although it is not covered by the federal vaccine procurement program, the state government has decided to procure it independently. “We think ultimately it would be good to have this on the national program right across Australia,” Picton said.
Broader Implications and Future Prospects
South Australia’s Chief Public Health Officer, Dr. Nicola Spurrier, highlighted the severity of flu complications in children. “The flu is not just a simple cold; children get really sick with it – high temperatures, ear infections, sometimes bronchiolitis,” she explained. In Clive’s case, the flu led to encephalopathy, an inflammation of the brain.
FluMist is registered for use in Australia for individuals aged two to 19, but the focus will be on younger children due to their higher hospitalization rates. “We’re absolutely thrilled that we’ve got this as an option now for parents going forward,” Dr. Spurrier remarked.
This development follows similar moves by the New South Wales and Queensland governments to introduce needle-free vaccines. It also coincides with advancements in an Australian-made needle-free vaccine patch called Vaxxas, which recently secured $90 million in funding to accelerate its development. This funding will support the installation of semi-automated manufacturing lines and later-stage clinical trials, promising a future with more accessible vaccination options.
The introduction of FluMist in South Australia represents a significant step towards improving public health and preventing severe flu cases among young children. As the state prepares to roll out this new vaccination program, the hope is that it will lead to higher vaccination rates and fewer hospitalizations, ultimately safeguarding the health of the youngest and most vulnerable members of the community.