Immune-checkpoint therapy (ICT), a groundbreaking approach that uses a patient’s immune system to combat cancer, has transformed oncology over the last two decades. However, a significant number of patients fail to respond to this therapy, with resistance often attributed to immune-evasive or “cold” tumors. The mechanisms behind this resistance have remained elusive until now.
According to Dr. Scott Lippman, Distinguished Professor of Medicine and academic director of the M.S. program in Precision Medicine at the University of California San Diego School of Medicine, “Identifying likely non-responders and mechanisms behind this resistance is crucial to guiding precision ICT, developing more effective personalized treatments, and avoiding severe costs of ineffective therapy.”
Dr. Lippman, also a member of UC San Diego’s Center for Engineering and Cancer and Moores Cancer Center, has led a new study published in the Journal of Thoracic Oncology. This research not only sheds light on the genetic factors contributing to ICT resistance but also sparks the development of a promising new cancer vaccine.
Genetic Foundations of Resistance
The study builds on previous research from Lippman’s lab, which identified specific genetic changes on the short arm of chromosome 9, known as 9p, as a major driver of ICT nonresponse in certain head and neck cancers. These findings were particularly significant in cancers associated with the human papillomavirus (HPV), the most common and lethal form of head and neck squamous cancer.
Loss of one or both copies of 9p was found to be the most profound driver of immune-evasion and ICT resistance across various cancers. This discovery has been corroborated by multiple research groups and extended to include subsets of lung, mesothelioma, melanoma, and bladder cancer.
“Our results were utilized by the nation’s largest clinical laboratories to support the Clinical Laboratory Improvement Amendments (CLIA) accuracy requirement in developing Medicare-covered 9p ICT-predictive tests,” Lippman noted, emphasizing the impact on standard clinical biomarker tests.
These tests are now applicable to other cancers exhibiting loss-of-9p immune-evasion, notably non-squamous non-small cell lung cancer. The 2021 study also highlighted the indirect consequences of 9p loss, particularly the reduction in cytokines such as CXCL9 and CXCL10, which are crucial for immune response.
The Role of Interferons
The new study delves deeper into the genetic underpinnings of ICT resistance, focusing on type-I interferon-I (IFN-I) genes located on chromosome 9p. Historically underappreciated in cancer research, these genes have now been identified as key players in immune evasion.
Dr. Teresa Davoli, co-senior author and associate professor at New York University’s Grossman School of Medicine, explained, “We found that IFN-I deficiency contributes to an immune-deserted state, making it harder for immune cells to infiltrate tumors and produce certain chemokines, primarily CXCL9/10.”
The research team conducted a comprehensive analysis involving large-scale genomic datasets, cell lines, and mouse models. Their findings revealed a direct correlation between tumor IFN-I status and the abundance of CXCL9/10+ immune cells, highlighting the significance of IFN-I gene losses at 9p21.
“These findings suggest that there is strong selective pressure for deep deletions of interferon genes, likely to drive tumor immune-evasion,” noted co-author Azhar Khandekar, emphasizing the evolutionary adaptations of cancer cells.
A New Vaccine on the Horizon
The implications of these findings are substantial, particularly for the development of personalized cancer vaccines. Unlike ICT, which generally releases the brakes on the immune system, cancer vaccines target specific tumor antigens or pathways.
Dr. Steve Dubinett, dean of the David Geffen School of Medicine at UC Los Angeles, remarked, “This report provides important new insights into the regulation of CXCL9/10, offering a level of granularity not previously described in the literature.”
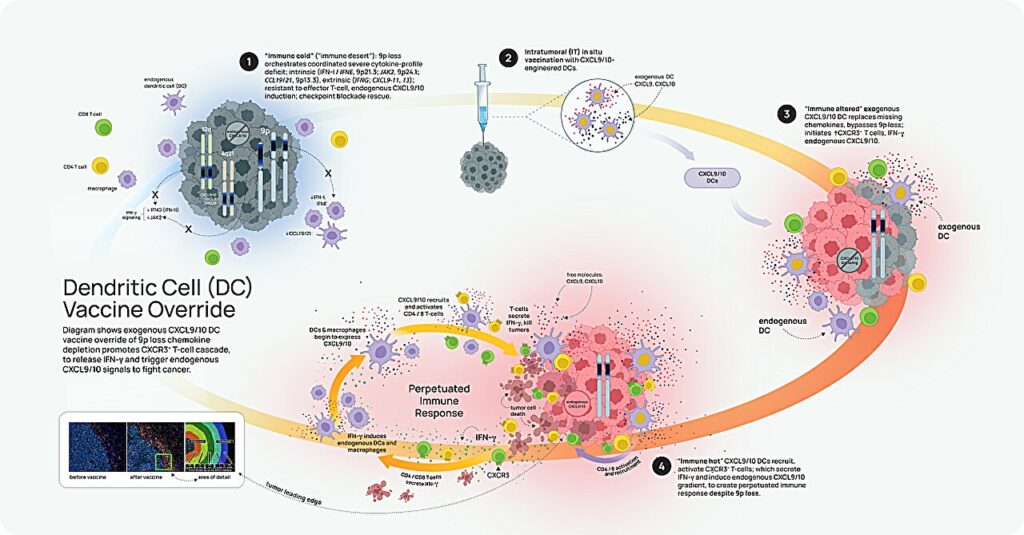
To translate these insights into clinical applications, Dubinett’s team has developed a vaccine using engineered dendritic cells (DC) to bypass CXCL9/10 depletion and trigger an anti-tumor immune response in 9p-loss tumors. Although this approach has shown promise in mice, further research is needed to assess its clinical potential.
“This is a huge step forward for personalized immune cancer therapy,” Dr. Lippman concluded, highlighting the potential of this breakthrough to transform cancer treatment.
As the scientific community continues to unravel the complexities of cancer resistance and immune response, the findings from this study offer hope for more effective and personalized treatment options. The development of a new cancer vaccine marks a significant milestone in the ongoing battle against cancer, promising a future where therapies are tailored to the genetic makeup of each patient’s tumor.







